Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NiO 纳米颗粒增强 TiO2 结构性能的作用及其在光降解和析氢中的应用
ACS Omega ( IF 3.7 ) Pub Date : 2021-11-03 , DOI: 10.1021/acsomega.1c03693
Mohammed A Mannaa 1 , Khaled F Qasim 2 , Fares T Alshorifi 3, 4 , Salah M El-Bahy 5 , Reda S Salama 6
Affiliation
|
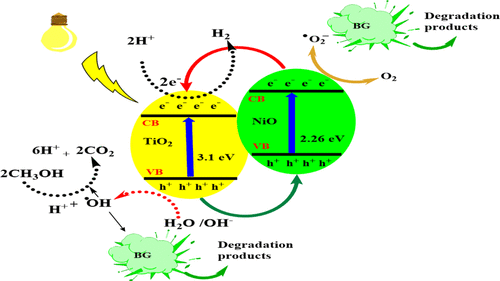
以十六烷基三甲基溴化铵为模板,通过表面活性剂辅助溶胶-凝胶法制备了不同 NiO 负载量(3-20.0 wt%)的纯介孔 TiO 2纳米粒子和改性介孔 TiO 2 纳米粒子。使用N 2吸附-脱附分析、能量色散光谱、扫描电子显微镜、透射电子显微镜、X射线衍射、X射线光电子能谱、紫外-可见光谱、傅里叶变换红外光谱检查了不同样品的光学和结构性质。光谱学和光致发光(PL)光谱学。 X射线衍射结果证实Ni 2+插入到TiO 2晶格中,并且添加NiO后晶粒尺寸显着减小。漫反射光谱吸收边出现明显红移,添加NiO后可见光区出现新的吸收带,表明表面缺陷和氧空位的形成。当NiO含量增加时,TiO 2的光学带隙急剧减小。使用 PL 光谱检查表面缺陷和氧空位的增加。研究了合成样品在可见光下光降解亮绿(BG)和苯酚以及产生氢气的光催化性能。 10% NiO/TiO 2表现出最高的光催化效率。由于NiO/TiO 2界面上p-n结的形成,光催化活性得到提高,有效促进了光生电子/空穴对的分离,从而增强了其光降解活性。 根据光催化活性结果,NiO含量被认为是影响BG和苯酚光降解以及H 2析出的最重要因素之一。此外,我们还讨论了 BG 和苯酚的光降解、矿化(总有机碳)和光催化反应动力学的机制。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号