当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structures of glycogen-debranching enzyme mutants in complex with oligosaccharides
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2021-11-02 , DOI: 10.1107/s2053230x21010918 Miaomiao Shen 1 , Xiaoxin Gong 1 , Song Xiang 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2021-11-02 , DOI: 10.1107/s2053230x21010918 Miaomiao Shen 1 , Xiaoxin Gong 1 , Song Xiang 1
Affiliation
|
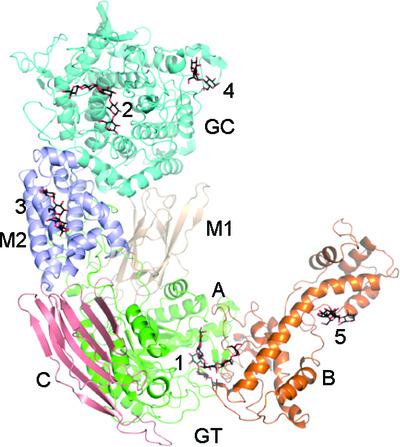
Debranching is a critical step in the mobilization of the important energy store glycogen. In eukaryotes, including fungi and animals, the highly conserved glycogen-debranching enzyme (GDE) debranches glycogen by a glucanotransferase (GT) reaction followed by a glucosidase (GC) reaction. Previous work indicated that these reactions are catalyzed by two active sites located more than 50 Å apart and provided insights into their catalytic mechanisms and substrate recognition. Here, five crystal structures of GDE in complex with oligosaccharides with 4–9 glucose residues are presented. The data suggest that the glycogen main chain plays a critical role in binding to the GT and GC active sites of GDE and that a minimum of five main-chain residues are required for optimal binding.
中文翻译:

与寡糖复合的糖原脱支酶突变体的晶体结构
脱支是重要能量储存糖原动员的关键步骤。在真核生物(包括真菌和动物)中,高度保守的糖原脱支酶 (GDE) 通过葡聚糖转移酶 (GT) 反应和随后的葡萄糖苷酶 (GC) 反应使糖原脱支。先前的工作表明,这些反应是由两个相距超过 50 Å 的活性位点催化的,并提供了对其催化机制和底物识别的见解。这里展示了 GDE 与具有 4-9 个葡萄糖残基的寡糖复合物的五种晶体结构。数据表明,糖原主链在与 GDE 的 GT 和 GC 活性位点的结合中起着关键作用,并且至少需要五个主链残基才能实现最佳结合。
更新日期:2021-11-03
中文翻译:

与寡糖复合的糖原脱支酶突变体的晶体结构
脱支是重要能量储存糖原动员的关键步骤。在真核生物(包括真菌和动物)中,高度保守的糖原脱支酶 (GDE) 通过葡聚糖转移酶 (GT) 反应和随后的葡萄糖苷酶 (GC) 反应使糖原脱支。先前的工作表明,这些反应是由两个相距超过 50 Å 的活性位点催化的,并提供了对其催化机制和底物识别的见解。这里展示了 GDE 与具有 4-9 个葡萄糖残基的寡糖复合物的五种晶体结构。数据表明,糖原主链在与 GDE 的 GT 和 GC 活性位点的结合中起着关键作用,并且至少需要五个主链残基才能实现最佳结合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号