Progress in Nuclear Magnetic Resonance Spectroscopy ( IF 7.3 ) Pub Date : 2021-11-02 , DOI: 10.1016/j.pnmrs.2021.10.001 Vitali Tugarinov 1 , Alberto Ceccon 1 , G Marius Clore 1
|
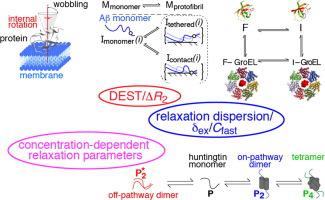
A survey, primarily based on work in the authors’ laboratory during the last 10 years, is provided of recent developments in NMR studies of exchange processes involving protein-ligand and protein-protein interactions. We start with a brief overview of the theoretical background of Dark state Exchange Saturation Transfer (DEST) and lifetime line-broadening (ΔR2) NMR methodology. Some limitations of the DEST/ΔR2 methodology in applications to molecular systems with intermediate molecular weights are discussed, along with the means of overcoming these limitations with the help of closely related exchange NMR techniques, such as the measurements of Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion, exchange-induced chemical shifts or rapidly-relaxing components of relaxation decays. Some theoretical underpinnings of the quantitative description of global dynamics of proteins on the surface of very high molecular weight particles (nanoparticles) are discussed. Subsequently, several applications of DEST/ΔR2 methodology are described from a methodological perspective with an emphasis on providing examples of how kinetic and relaxation parameters for exchanging systems can be reliably extracted from NMR data for each particular model of exchange. Among exchanging systems that are not associated with high molecular weight species, we describe several exchange NMR-based studies that focus on kinetic modelling of transient pre-nucleation oligomerization of huntingtin peptides that precedes aggregation and fibril formation.
中文翻译:

用于探索配体结合和蛋白质-蛋白质相互作用中“暗”状态的 NMR 方法
一项主要基于作者实验室过去 10 年工作的调查提供了 NMR 研究中涉及蛋白质-配体和蛋白质-蛋白质相互作用的交换过程的最新进展。我们首先简要概述暗状态交换饱和转移 (DEST) 和寿命线展宽 (Δ R 2 ) NMR 方法的理论背景。DEST/Δ R 2的一些限制讨论了应用于中等分子量分子系统的方法,以及借助密切相关的交换 NMR 技术克服这些限制的方法,例如测量 Carr-Purcell-Meiboom-Gill (CPMG) 弛豫色散、交换-诱导的化学位移或弛豫衰减的快速弛豫成分。讨论了定量描述超高分子量粒子(纳米粒子)表面蛋白质的全局动力学的一些理论基础。随后,DEST/Δ R 2的几次应用方法论是从方法学的角度描述的,重点是提供如何从每个特定交换模型的 NMR 数据中可靠地提取交换系统的动力学和弛豫参数的示例。在与高分子量物种无关的交换系统中,我们描述了几项基于交换 NMR 的研究,这些研究侧重于在聚集和原纤维形成之前对亨廷顿肽的瞬时预成核寡聚化的动力学建模。






























 京公网安备 11010802027423号
京公网安备 11010802027423号