当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the Oxidation State of Cu Electrodes for Selective Electrosynthesis of Ammonia from Nitrate
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-11-01 , DOI: 10.1021/acsami.1c10946
Jili Yuan 1, 2 , Zhuo Xing 3 , Yanhong Tang 1 , Chengbin Liu 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-11-01 , DOI: 10.1021/acsami.1c10946
Jili Yuan 1, 2 , Zhuo Xing 3 , Yanhong Tang 1 , Chengbin Liu 2
Affiliation
|
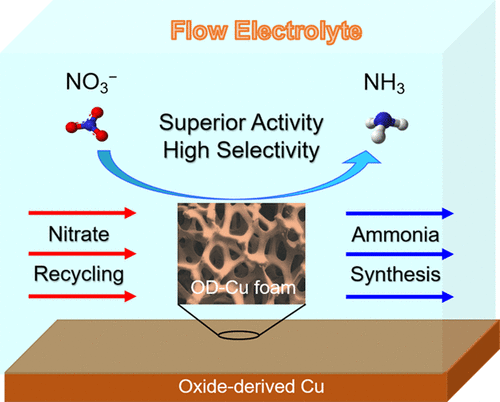
Electrochemical reduction of nitrate (NO3–) to ammonia (NH3) provides a promising route for recycling nitrate from wastewater to balance the nitrogen cycle and sustainable production of ammonia. Among various catalytic materials for NO3– electroreduction, Cu shows a favorable selectivity to NH3. However, Cu can be easily oxidized, while the effect of the Cu oxidation state on NO3– reduction remains to be elucidated. Here, we report that oxidic Cu formed on a Cu electrode can enhance its activity and selectivity for NO3– reduction to NH3. We first used a polished Cu foil as a model catalyst for NO3– reduction and found that a brief exposure of the Cu electrode to air could increase its yield rate and Faradaic efficiency for NH3 production. The improved catalytic performance was attributed to the formed Cu+ sites that can reduce the energy barrier for NO3– reduction to NH3 and suppress the competing HER reaction. Based on this finding, an oxide-derived Cu (OD-Cu) electrode was prepared by annealing a Cu foil in O2 gas followed by electroreduction, which exhibited superior performance for NO3– reduction to NH3, with a Faradaic efficiency of 92% and a yield rate of 1.1 mmol h–1 cm–2 for NH3 production at −0.15 V versus reversible hydrogen electrode. Moreover, an OD-Cu foam electrode was similarly developed to demonstrate NO3– recycling from a low-concentration NO3– solution, which showed a nearly 100% conversion of NO3– to NH3 using a circulating flow cell.
中文翻译:

调节用于从硝酸盐中选择性电合成氨的 Cu 电极的氧化状态
硝酸盐 (NO 3 – )电化学还原为氨 (NH 3 ) 为从废水中回收硝酸盐以平衡氮循环和氨的可持续生产提供了一条有前途的途径。在用于各种NO催化材料3 -电还原,铜示出有利的选择性,以NH 3。然而,Cu很容易被氧化,而Cu氧化态对NO 3 -还原的影响仍有待阐明。在这里,我们报告形成于铜电极可以增强其对NO的活性和选择性是氧化铜3 -还原为NH 3。我们首先使用抛光的铜箔作为 NO 的模型催化剂3 –还原,发现将 Cu 电极短暂暴露在空气中可以提高其用于 NH 3生产的产率和法拉第效率。改进的催化性能归因于形成的 Cu +位点,该位点可以降低 NO 3 –还原为 NH 3的能垒并抑制竞争性 HER 反应。基于这一发现,通过在 O 2气体中对铜箔进行退火然后进行电还原制备了氧化物衍生的 Cu (OD-Cu) 电极,该电极对 NO 3 -还原为 NH 3表现出优异的性能,法拉第效率为 92 % 和 1.1 mmol h –1的产率cm –2对于在 -0.15 V 的NH 3生产与可逆氢电极。而且,OD-Cu系泡沫电极同样地开发以证明NO 3 -从低浓度NO再循环3 -溶液,这表明NO的几乎100%的转化率3 -至NH 3使用循环流动池。
更新日期:2021-11-10
中文翻译:

调节用于从硝酸盐中选择性电合成氨的 Cu 电极的氧化状态
硝酸盐 (NO 3 – )电化学还原为氨 (NH 3 ) 为从废水中回收硝酸盐以平衡氮循环和氨的可持续生产提供了一条有前途的途径。在用于各种NO催化材料3 -电还原,铜示出有利的选择性,以NH 3。然而,Cu很容易被氧化,而Cu氧化态对NO 3 -还原的影响仍有待阐明。在这里,我们报告形成于铜电极可以增强其对NO的活性和选择性是氧化铜3 -还原为NH 3。我们首先使用抛光的铜箔作为 NO 的模型催化剂3 –还原,发现将 Cu 电极短暂暴露在空气中可以提高其用于 NH 3生产的产率和法拉第效率。改进的催化性能归因于形成的 Cu +位点,该位点可以降低 NO 3 –还原为 NH 3的能垒并抑制竞争性 HER 反应。基于这一发现,通过在 O 2气体中对铜箔进行退火然后进行电还原制备了氧化物衍生的 Cu (OD-Cu) 电极,该电极对 NO 3 -还原为 NH 3表现出优异的性能,法拉第效率为 92 % 和 1.1 mmol h –1的产率cm –2对于在 -0.15 V 的NH 3生产与可逆氢电极。而且,OD-Cu系泡沫电极同样地开发以证明NO 3 -从低浓度NO再循环3 -溶液,这表明NO的几乎100%的转化率3 -至NH 3使用循环流动池。































 京公网安备 11010802027423号
京公网安备 11010802027423号