当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triglyceride-Mimetic Structure-Gated Prodrug Nanoparticles for Smart Cancer Therapy
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-11-01 , DOI: 10.1021/acs.jmedchem.1c01328 Chutong Tian 1 , Jingjing Guo 1 , Yifan Miao 1 , Shunzhe Zheng 1 , Bingjun Sun 1 , Mengchi Sun 1 , Qing Ye 1 , Wenxue Liu 2 , Shuang Zhou 1 , Ken-Ichiro Kamei 1, 3 , Zhonggui He 1 , Jin Sun 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-11-01 , DOI: 10.1021/acs.jmedchem.1c01328 Chutong Tian 1 , Jingjing Guo 1 , Yifan Miao 1 , Shunzhe Zheng 1 , Bingjun Sun 1 , Mengchi Sun 1 , Qing Ye 1 , Wenxue Liu 2 , Shuang Zhou 1 , Ken-Ichiro Kamei 1, 3 , Zhonggui He 1 , Jin Sun 1
Affiliation
|
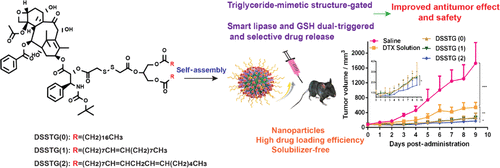
Off-target drug release and insufficient drug delivery are the main obstacles for effective anticancer chemotherapy. Prodrug-based self-assembled nanoparticles bioactivated under tumor-specific conditions are one of the effective strategies to achieve on-demand drug release and effective tumor accumulation. Herein, stimuli-activable prodrugs are designed yielding smart tumor delivery by combination of the triglyceride-mimic (TG-mimetic) prodrug structure and disulfide bond. Surprisingly, these prodrugs can self-assemble into uniform nanoparticles (NPs) with a high drug loading (over 40%) and accumulate in tumor sites specifically. The super hydrophobic TG structure can act as a gate that senses lipase to selectively control over NP dissociation and affect the glutathione-triggered prodrug activation. In addition, the impacts of the double bonds in the prodrug NPs on parent drug release and the following cytotoxicity, pharmacokinetics, and antitumor efficiency are further demonstrated. Our findings highlight the promising potential of TG-mimetic structure-gated prodrug nanoparticles for tumor-specific drug delivery.
中文翻译:

用于智能癌症治疗的甘油三酯模拟结构门控前药纳米粒子
脱靶药物释放和药物递送不足是有效抗癌化疗的主要障碍。在肿瘤特异性条件下生物活化的基于前体药物的自组装纳米粒子是实现按需药物释放和有效肿瘤积累的有效策略之一。在此,可刺激激活的前药被设计成通过甘油三酯模拟(TG-模拟)前药结构和二硫键的组合产生智能肿瘤递送。令人惊讶的是,这些前药可以自组装成具有高载药量(超过 40%)的均匀纳米颗粒 (NPs),并专门在肿瘤部位积累。超疏水的 TG 结构可以充当感应脂肪酶的门,以选择性地控制 NP 解离并影响谷胱甘肽触发的前药活化。此外,进一步证明了前药纳米颗粒中双键对母体药物释放的影响以及随后的细胞毒性、药代动力学和抗肿瘤效率。我们的研究结果突出了 TG 模拟结构门控前药纳米粒子在肿瘤特异性药物递送中的巨大潜力。
更新日期:2021-11-11
中文翻译:

用于智能癌症治疗的甘油三酯模拟结构门控前药纳米粒子
脱靶药物释放和药物递送不足是有效抗癌化疗的主要障碍。在肿瘤特异性条件下生物活化的基于前体药物的自组装纳米粒子是实现按需药物释放和有效肿瘤积累的有效策略之一。在此,可刺激激活的前药被设计成通过甘油三酯模拟(TG-模拟)前药结构和二硫键的组合产生智能肿瘤递送。令人惊讶的是,这些前药可以自组装成具有高载药量(超过 40%)的均匀纳米颗粒 (NPs),并专门在肿瘤部位积累。超疏水的 TG 结构可以充当感应脂肪酶的门,以选择性地控制 NP 解离并影响谷胱甘肽触发的前药活化。此外,进一步证明了前药纳米颗粒中双键对母体药物释放的影响以及随后的细胞毒性、药代动力学和抗肿瘤效率。我们的研究结果突出了 TG 模拟结构门控前药纳米粒子在肿瘤特异性药物递送中的巨大潜力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号