当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Catalysis Using Boronic Acid and Chiral Amine: Acyclic Quaternary Carbons via Enantioselective Alkylation of Branched Aldehydes with Allylic Alcohols
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-17 , DOI: 10.1021/jacs.6b06101 Xiaobin Mo 1 , Dennis G. Hall 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-17 , DOI: 10.1021/jacs.6b06101 Xiaobin Mo 1 , Dennis G. Hall 1
Affiliation
|
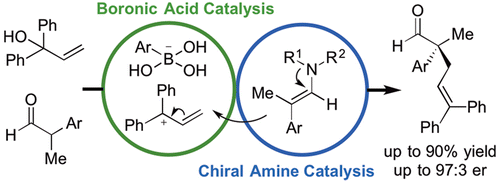
A ferrocenium boronic acid salt activates allylic alcohols to generate transient carbocations that react with in situ-generated chiral enamines from branched aldehydes. The optimized conditions afford the desired acyclic products embedding a methyl-aryl quaternary carbon center with up to 90% yield and 97:3 enantiomeric ratio, with only water as the byproduct. This noble-metal-free method complements alternative methods that are incompatible with carbon-halogen bonds and other sensitive functional groups.
中文翻译:

使用硼酸和手性胺的双催化:通过支链醛与烯丙醇对映选择性烷基化的无环季碳
二茂铁硼酸盐活化烯丙醇以产生瞬时碳阳离子,该碳阳离子与从支化醛原位生成的手性烯胺反应。优化的条件提供了嵌入甲基-芳基季碳中心的所需无环产物,产率高达 90%,对映体比为 97:3,只有水作为副产物。这种不含贵金属的方法补充了与碳卤键和其他敏感官能团不兼容的替代方法。
更新日期:2016-08-17
中文翻译:

使用硼酸和手性胺的双催化:通过支链醛与烯丙醇对映选择性烷基化的无环季碳
二茂铁硼酸盐活化烯丙醇以产生瞬时碳阳离子,该碳阳离子与从支化醛原位生成的手性烯胺反应。优化的条件提供了嵌入甲基-芳基季碳中心的所需无环产物,产率高达 90%,对映体比为 97:3,只有水作为副产物。这种不含贵金属的方法补充了与碳卤键和其他敏感官能团不兼容的替代方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号