当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An ROS-Activatable Nanoassembly Remodulates Tumor Cell Metabolism for Enhanced Ferroptosis Therapy
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-10-28 , DOI: 10.1002/adhm.202101702
Yuchen Zhang 1 , Liqi Li 2 , Yanan Li 1 , Yang Fei 1 , Chencheng Xue 1 , Xuemei Yao 1 , Youbo Zhao 1 , Xuan Wang 1 , Menghuan Li 1 , Zhong Luo 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-10-28 , DOI: 10.1002/adhm.202101702
Yuchen Zhang 1 , Liqi Li 2 , Yanan Li 1 , Yang Fei 1 , Chencheng Xue 1 , Xuemei Yao 1 , Youbo Zhao 1 , Xuan Wang 1 , Menghuan Li 1 , Zhong Luo 1
Affiliation
|
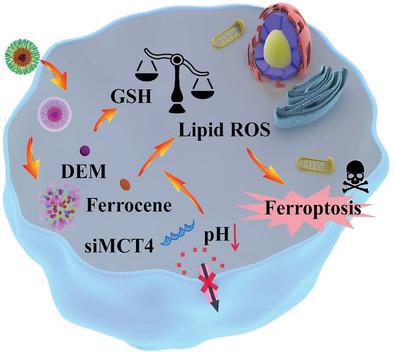
Ferroptosis is an emerging antitumor option and has demonstrated unique advantages against many tumor indications. However, its efficacy is potentially hindered by the endogenous lipid peroxide-scavenging mechanisms and the reliance on acidic pH. Herein, a nanointegrated strategy based on clinically-safe components to synergistically remodel glutathione and lactate metabolism in tumor cells for enhanced ferroptosis therapy is developed. First ferrocene is conjugated on PEGylated polyamidoamine dendrimers via reactive oxygen species (ROS)-cleavable thioketal linkage, which would further self-assemble with the glutathione (GSH)-depleting agent diethyl maleate (DEM) and monocarboxylate transporter 4-inhibiting siRNA (siMCT4) to afford biostable nanoassemblies (siMCT4-PAMAM-PEG-TK-Fc@DEM). The nanoassemblies can be activated by the elevated ROS levels in tumor intracellular environment and readily release the incorporated therapeutic contents, afterward DEM can directly conjugate to GSH to disrupt the glutathione peroxidase 4 (GPX4)-mediated antioxidant defense, while siMCT4 can block the MCT4-mediated efflux of lactic acid and acidify the intracellular milieu, both of which can improve the ferrocene-catalyzed lipid peroxidation and induce pronounced ferroptotic damage. The siMCT4-PAMAM-PEG-TK-Fc@DEM nanoplatform demonstrates high ferroptosis-based antitumor potency and good biocompatibility in vitro and in vivo, which may offer new avenues for the development of more advanced antitumor therapeutics with improved translatability.
中文翻译:

可激活 ROS 的纳米组件可调节肿瘤细胞代谢以增强铁死亡治疗
Ferroptosis 是一种新兴的抗肿瘤选择,并在许多肿瘤适应症方面表现出独特的优势。然而,其功效可能受到内源性脂质过氧化物清除机制和对酸性 pH 值的依赖的阻碍。在此,开发了一种基于临床安全成分的纳米整合策略,以协同重塑肿瘤细胞中的谷胱甘肽和乳酸代谢,以增强铁死亡治疗。第一个二茂铁通过活性氧 (ROS) 可切割的硫缩酮键与聚乙二醇化的聚酰胺胺树枝状大分子结合,这将进一步与谷胱甘肽 (GSH) 消耗剂马来酸二乙酯 (DEM) 和单羧酸转运蛋白 4 抑制 siRNA (siMCT4) 自组装提供生物稳定的纳米组件(siMCT4-PAMAM-PEG-TK-Fc@DEM)。纳米组件可以被肿瘤细胞内环境中升高的 ROS 水平激活并容易释放掺入的治疗成分,然后 DEM 可以直接与 GSH 结合以破坏谷胱甘肽过氧化物酶 4 (GPX4) 介导的抗氧化防御,而 siMCT4 可以阻断 MCT4-介导的乳酸流出和酸化细胞内环境,这两者都可以改善二茂铁催化的脂质过氧化并诱导明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高的基于铁死亡的抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。之后 DEM 可以直接与 GSH 结合,破坏谷胱甘肽过氧化物酶 4 (GPX4) 介导的抗氧化防御,而 siMCT4 可以阻断 MCT4 介导的乳酸流出并酸化细胞内环境,两者都可以改善二茂铁催化的脂质过氧化并引起明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高的基于铁死亡的抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。之后 DEM 可以直接与 GSH 结合,破坏谷胱甘肽过氧化物酶 4 (GPX4) 介导的抗氧化防御,而 siMCT4 可以阻断 MCT4 介导的乳酸流出并酸化细胞内环境,两者都可以改善二茂铁催化的脂质过氧化并引起明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高的基于铁死亡的抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。两者都可以改善二茂铁催化的脂质过氧化并诱导明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高铁死亡抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。两者都可以改善二茂铁催化的脂质过氧化并诱导明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高铁死亡抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。
更新日期:2021-10-28
中文翻译:

可激活 ROS 的纳米组件可调节肿瘤细胞代谢以增强铁死亡治疗
Ferroptosis 是一种新兴的抗肿瘤选择,并在许多肿瘤适应症方面表现出独特的优势。然而,其功效可能受到内源性脂质过氧化物清除机制和对酸性 pH 值的依赖的阻碍。在此,开发了一种基于临床安全成分的纳米整合策略,以协同重塑肿瘤细胞中的谷胱甘肽和乳酸代谢,以增强铁死亡治疗。第一个二茂铁通过活性氧 (ROS) 可切割的硫缩酮键与聚乙二醇化的聚酰胺胺树枝状大分子结合,这将进一步与谷胱甘肽 (GSH) 消耗剂马来酸二乙酯 (DEM) 和单羧酸转运蛋白 4 抑制 siRNA (siMCT4) 自组装提供生物稳定的纳米组件(siMCT4-PAMAM-PEG-TK-Fc@DEM)。纳米组件可以被肿瘤细胞内环境中升高的 ROS 水平激活并容易释放掺入的治疗成分,然后 DEM 可以直接与 GSH 结合以破坏谷胱甘肽过氧化物酶 4 (GPX4) 介导的抗氧化防御,而 siMCT4 可以阻断 MCT4-介导的乳酸流出和酸化细胞内环境,这两者都可以改善二茂铁催化的脂质过氧化并诱导明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高的基于铁死亡的抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。之后 DEM 可以直接与 GSH 结合,破坏谷胱甘肽过氧化物酶 4 (GPX4) 介导的抗氧化防御,而 siMCT4 可以阻断 MCT4 介导的乳酸流出并酸化细胞内环境,两者都可以改善二茂铁催化的脂质过氧化并引起明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高的基于铁死亡的抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。之后 DEM 可以直接与 GSH 结合,破坏谷胱甘肽过氧化物酶 4 (GPX4) 介导的抗氧化防御,而 siMCT4 可以阻断 MCT4 介导的乳酸流出并酸化细胞内环境,两者都可以改善二茂铁催化的脂质过氧化并引起明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高的基于铁死亡的抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。两者都可以改善二茂铁催化的脂质过氧化并诱导明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高铁死亡抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。两者都可以改善二茂铁催化的脂质过氧化并诱导明显的铁死亡损伤。siMCT4-PAMAM-PEG-TK-Fc@DEM 纳米平台在体外和体内表现出高铁死亡抗肿瘤效力和良好的生物相容性,这可能为开发更先进的抗肿瘤疗法提供新的途径,并提高可转化性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号