当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ascendancy of Nitrogen Heterocycles in the Computationally Designed Mn(I)PNN Pincer Catalysts on the Hydrogenation of Carbon Dioxide to Methanol
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-10-29 , DOI: 10.1021/acs.inorgchem.1c02689
Vidya D Avasare 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-10-29 , DOI: 10.1021/acs.inorgchem.1c02689
Vidya D Avasare 1
Affiliation
|
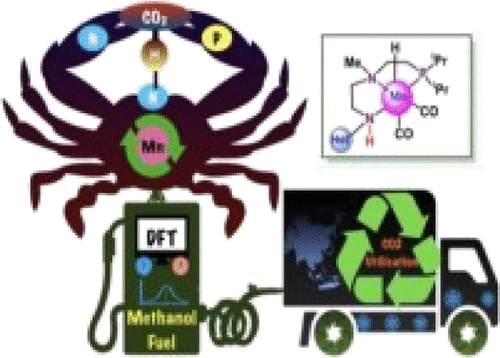
The development of sustainable catalysts to get methanol from CO2 under milder conditions and without any additives is still considered an arduous task. In many instances, transition-metal-catalyzed carbon dioxide to formic acid formation is more facile than methanol formation. This article provides comprehensive density functional theoretic investigations of six new Mn(I)PNN complexes, which are designed to perform CO2 to methanol conversion under milder reaction conditions. All these six catalysts have similar structural features except at terminal nitrogen, −N (1), where adenine-inspired nitrogen heterocycles containing pyridine and pyrimidine moieties are attached to instill an electron withdrawing effect on the central metal and thus to facilitate dihydrogen polarization during the catalyst regeneration. All these computationally modeled Mn(I)PNN complexes demonstrate the promising catalytic activity to get methanol through cascade catalytic cycles at 298.15 K. The metal–ligand cooperative (MLC) as well as noncooperative (NC) pathways are investigated for each catalytic cycle. The NC pathway is the preferred pathway for formic acid and formaldehyde formation, whereas methanol formation proceeds through only the MLC pathway. Different nitrogen heterocycles attached to the −N (1) terminal manifested a considerable amount of impact on the Gibbs free energies, overall activation energies, and computed turnover frequencies (TOFs). Among all the catalysts, SPCAT02 provides excellent TOFs for HCO2H (500 151 h–1), HCHO (11 912 h–1), and CH3OH (2 372 400 h–1) formation at 50 °C. SPCAT04 is found to be a better catalyst for the selective formation of formic acid formation at room temperature than the rest of the catalysts. The computed TOF results are found reliable upon comparison with experimentally established catalysts. To establish the structure–activity relationship, the activation strain model and Fukui function calculations are performed on all the catalysts. Both these studies provide complementary results. The present study revealed a very important finding that a more electrophilic metal center could facilitate the CO2 hydrogenation reaction robustly. All computationally designed catalysts could be cheaper and better alternatives to convert CO2 to methanol under mild reaction conditions in an aqueous medium.
中文翻译:

计算设计的 Mn(I)PNN 钳形催化剂中氮杂环在二氧化碳加氢制甲醇中的优势
在更温和的条件下,不使用任何添加剂,从 CO 2中提取甲醇的可持续催化剂的开发仍被认为是一项艰巨的任务。在许多情况下,过渡金属催化的二氧化碳形成甲酸比形成甲醇更容易。本文提供了六种新的 Mn(I)PNN 配合物的综合密度泛函理论研究,这些配合物旨在执行 CO 2在较温和的反应条件下转化为甲醇。所有这六种催化剂都具有相似的结构特征,除了末端氮,-N(1),其中含有吡啶和嘧啶部分的腺嘌呤启发的氮杂环被连接以在中心金属上注入吸电子效应,从而促进二氢极化过程中的极化。催化剂再生。所有这些计算建模的 Mn(I)PNN 配合物都证明了在 298.15 K 下通过级联催化循环获得甲醇的有希望的催化活性。针对每个催化循环研究了金属-配体协同 (MLC) 以及非协同 (NC) 途径。NC 途径是甲酸和甲醛形成的首选途径,而甲醇形成仅通过 MLC 途径进行。连接到 -N (1) 末端的不同氮杂环对吉布斯自由能、总活化能和计算的转换频率 (TOF) 表现出相当大的影响。在所有催化剂中,SPCAT02为 HCO 2 H (500 151 h –1 )、HCHO (11 912 h –1 ) 和 CH 3 OH (2 372 400 h –1 ) 在 50 °C 的形成提供了出色的 TOF。SPCAT04发现在室温下选择性形成甲酸的催化剂比其他催化剂更好。通过与实验建立的催化剂进行比较,发现计算的 TOF 结果是可靠的。为了建立构效关系,对所有催化剂进行了活化应变模型和福井函数计算。这两项研究都提供了互补的结果。本研究揭示了一个非常重要的发现,即更亲电子的金属中心可以有力地促进CO 2加氢反应。所有计算设计的催化剂都可能是在水介质中温和反应条件下将 CO 2转化为甲醇的更便宜和更好的替代品。
更新日期:2021-10-29
中文翻译:

计算设计的 Mn(I)PNN 钳形催化剂中氮杂环在二氧化碳加氢制甲醇中的优势
在更温和的条件下,不使用任何添加剂,从 CO 2中提取甲醇的可持续催化剂的开发仍被认为是一项艰巨的任务。在许多情况下,过渡金属催化的二氧化碳形成甲酸比形成甲醇更容易。本文提供了六种新的 Mn(I)PNN 配合物的综合密度泛函理论研究,这些配合物旨在执行 CO 2在较温和的反应条件下转化为甲醇。所有这六种催化剂都具有相似的结构特征,除了末端氮,-N(1),其中含有吡啶和嘧啶部分的腺嘌呤启发的氮杂环被连接以在中心金属上注入吸电子效应,从而促进二氢极化过程中的极化。催化剂再生。所有这些计算建模的 Mn(I)PNN 配合物都证明了在 298.15 K 下通过级联催化循环获得甲醇的有希望的催化活性。针对每个催化循环研究了金属-配体协同 (MLC) 以及非协同 (NC) 途径。NC 途径是甲酸和甲醛形成的首选途径,而甲醇形成仅通过 MLC 途径进行。连接到 -N (1) 末端的不同氮杂环对吉布斯自由能、总活化能和计算的转换频率 (TOF) 表现出相当大的影响。在所有催化剂中,SPCAT02为 HCO 2 H (500 151 h –1 )、HCHO (11 912 h –1 ) 和 CH 3 OH (2 372 400 h –1 ) 在 50 °C 的形成提供了出色的 TOF。SPCAT04发现在室温下选择性形成甲酸的催化剂比其他催化剂更好。通过与实验建立的催化剂进行比较,发现计算的 TOF 结果是可靠的。为了建立构效关系,对所有催化剂进行了活化应变模型和福井函数计算。这两项研究都提供了互补的结果。本研究揭示了一个非常重要的发现,即更亲电子的金属中心可以有力地促进CO 2加氢反应。所有计算设计的催化剂都可能是在水介质中温和反应条件下将 CO 2转化为甲醇的更便宜和更好的替代品。

































 京公网安备 11010802027423号
京公网安备 11010802027423号