当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two-step, regioselective, multigram-scale synthesis of 2-(trifluoromethyl)indoles from 2-nitrotoluenes
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-10-29 , DOI: 10.1016/j.tetlet.2021.153515
Magdalena Walewska-Królikiewicz 1 , Bogdan Wilk 2 , Andrzej Kwast 1 , Zbigniew Wróbel 1
中文翻译:

从 2-硝基甲苯中两步、区域选择性、多克级合成 2-(三氟甲基)吲哚
更新日期:2021-12-03
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-10-29 , DOI: 10.1016/j.tetlet.2021.153515
Magdalena Walewska-Królikiewicz 1 , Bogdan Wilk 2 , Andrzej Kwast 1 , Zbigniew Wróbel 1
Affiliation
|
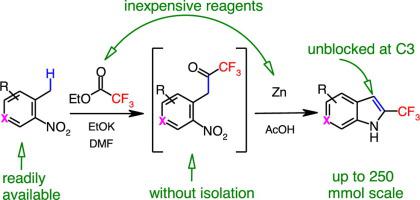
The acylation of 2-nitrotoluenes using ethyl trifluoroacetate, one of the least expensive sources of a trifluoromethyl group, produces intermediate 2-nitrobenzyl trifluoromethyl ketones which, without the need for isolation, are cyclized under the action of Zn/AcOH providing 2-(trifluoromethyl)indoles unsubstituted at the C3 position. The developed method avoids the use of expensive catalysts and harsh reaction conditions. The method is readily scalable without a significant decrease in performance upon going from 10- to 250-mmol scale.
中文翻译:

从 2-硝基甲苯中两步、区域选择性、多克级合成 2-(三氟甲基)吲哚
使用三氟乙酸乙酯(三氟甲基的最便宜来源之一)酰化 2-硝基甲苯,产生中间体 2-硝基苄基三氟甲基酮,无需分离,在 Zn/AcOH 的作用下环化,提供 2-(三氟甲基) )在 C3 位置未取代的吲哚。所开发的方法避免了使用昂贵的催化剂和苛刻的反应条件。该方法易于扩展,性能从 10 毫摩尔到 250 毫摩尔不会显着降低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号