当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of SM-130686 Based on the Development of Asymmetric Cu(I)F Catalysis To Access 2-Oxindoles Containing a Tetrasubstituted Carbon
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-05-27 , DOI: 10.1021/ja901995a Daisuke Tomita 1 , Kenzo Yamatsugu 1 , Motomu Kanai 1 , Masakatsu Shibasaki 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-05-27 , DOI: 10.1021/ja901995a Daisuke Tomita 1 , Kenzo Yamatsugu 1 , Motomu Kanai 1 , Masakatsu Shibasaki 1
Affiliation
|
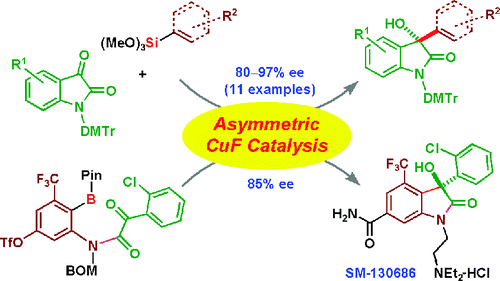
Two different catalytic enantioselective approaches to 3-aryl- and 3-alkenyl-3-hydroxy-2-oxindoles have been developed. First, enantioselective arylation and alkenylation reactions of isatins using aryltrimethoxysilanes and alkenyltrimethoxysilanes as nucleophiles can be catalyzed by a complex of CuF with structurally tuned Taniaphos (6) in the presence of a catalytic amount of ZnF(2). Despite the wide substrate scope, this intermolecular reaction was not applicable to a catalytic enantioselective synthesis of SM-130686 (1), a highly potent, orally active growth hormone secretagogue containing a sterically congested chiral tetrasubstituted carbon. Therefore, we developed an intramolecular catalytic enantioselective arylation of alpha-keto amides, taking advantage of the robustness of arylboronate reagents under multiple synthetic conversions and silica gel column chromatography purification. A complex of CuF with Ph-BPE (12) catalyzed the enantioselective arylation of alpha-keto amide 19, affording product 20 in 85% ee. The addition of ZnF(2) to this intramolecular reaction was not necessary. The first enantioselective synthesis of SM-130686 was achieved using this catalytic methodology. Because 2-oxyindoles are a versatile motif for biologically active compounds, the two types of Cu-catalyzed asymmetric reactions developed here will be useful for the synthesis of other natural products and pharmaceutical leads.
中文翻译:

SM-130686 的对映选择性合成基于不对称 Cu(I)F 催化获得含四取代碳的 2-羟吲哚
已经开发了两种不同的催化对映选择性方法来处理 3-芳基-和 3-烯基-3-羟基-2-羟吲哚。首先,使用芳基三甲氧基硅烷和烯基三甲氧基硅烷作为亲核试剂的靛红的对映选择性芳基化和烯基化反应可以由 CuF 与结构调整的 Taniaphos (6) 的复合物在催化量的 ZnF(2) 存在下催化。尽管底物范围很广,但这种分子间反应不适用于 SM-130686 (1) 的催化对映选择性合成,SM-130686 (1) 是一种含有空间拥挤的手性四取代碳的高效口服活性生长激素促分泌剂。因此,我们开发了α-酮酰胺的分子内催化对映选择性芳基化,利用芳基硼酸酯试剂在多次合成转化和硅胶柱色谱纯化下的稳定性。CuF 与 Ph-BPE (12) 的复合物催化 α-酮酰胺 19 的对映选择性芳基化,以 85% ee 提供产物 20。添加 ZnF(2) 到此分子内反应是没有必要的。使用这种催化方法实现了 SM-130686 的首次对映选择性合成。由于 2-羟基吲哚是生物活性化合物的通用基序,这里开发的两种铜催化不对称反应将可用于合成其他天然产物和药物先导化合物。添加 ZnF(2) 到此分子内反应是没有必要的。使用这种催化方法实现了 SM-130686 的首次对映选择性合成。由于 2-羟基吲哚是生物活性化合物的通用基序,这里开发的两种铜催化不对称反应将可用于合成其他天然产物和药物先导化合物。添加 ZnF(2) 到此分子内反应是没有必要的。使用这种催化方法实现了 SM-130686 的首次对映选择性合成。由于 2-羟基吲哚是生物活性化合物的通用基序,这里开发的两种铜催化不对称反应将可用于合成其他天然产物和药物先导化合物。
更新日期:2009-05-27
中文翻译:

SM-130686 的对映选择性合成基于不对称 Cu(I)F 催化获得含四取代碳的 2-羟吲哚
已经开发了两种不同的催化对映选择性方法来处理 3-芳基-和 3-烯基-3-羟基-2-羟吲哚。首先,使用芳基三甲氧基硅烷和烯基三甲氧基硅烷作为亲核试剂的靛红的对映选择性芳基化和烯基化反应可以由 CuF 与结构调整的 Taniaphos (6) 的复合物在催化量的 ZnF(2) 存在下催化。尽管底物范围很广,但这种分子间反应不适用于 SM-130686 (1) 的催化对映选择性合成,SM-130686 (1) 是一种含有空间拥挤的手性四取代碳的高效口服活性生长激素促分泌剂。因此,我们开发了α-酮酰胺的分子内催化对映选择性芳基化,利用芳基硼酸酯试剂在多次合成转化和硅胶柱色谱纯化下的稳定性。CuF 与 Ph-BPE (12) 的复合物催化 α-酮酰胺 19 的对映选择性芳基化,以 85% ee 提供产物 20。添加 ZnF(2) 到此分子内反应是没有必要的。使用这种催化方法实现了 SM-130686 的首次对映选择性合成。由于 2-羟基吲哚是生物活性化合物的通用基序,这里开发的两种铜催化不对称反应将可用于合成其他天然产物和药物先导化合物。添加 ZnF(2) 到此分子内反应是没有必要的。使用这种催化方法实现了 SM-130686 的首次对映选择性合成。由于 2-羟基吲哚是生物活性化合物的通用基序,这里开发的两种铜催化不对称反应将可用于合成其他天然产物和药物先导化合物。添加 ZnF(2) 到此分子内反应是没有必要的。使用这种催化方法实现了 SM-130686 的首次对映选择性合成。由于 2-羟基吲哚是生物活性化合物的通用基序,这里开发的两种铜催化不对称反应将可用于合成其他天然产物和药物先导化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号