Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-10-28 , DOI: 10.1016/j.colsurfa.2021.127822 J. Lazrak 1 , E. Ech-chihbi 1 , B. El Ibrahimi 2, 3 , F. El Hajjaji 1 , Z. Rais 1 , M. Tachihante 1 , M. Taleb 1
|
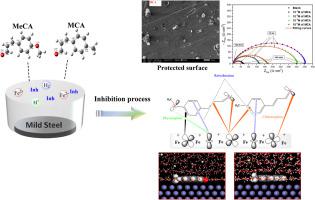
Corrosion prevention in mild steel (MS) is a major issue in the industry. The development of an effective protection strategy is a popular research area. In this work, two molecules, namely methoxy cinnamaldehyde (MCA) and methyl cinnamaldehyde (MeCA) were evaluated as corrosion inhibitors for mild steel in 1.0 M HCl solution, providing a strong basis for the development of new inhibitors platform in the field of corrosion. The corrosion inhibition performance of MCA and MeCA has been studied by using weight loss, Impedance Spectroscopy (EIS), Potentiodynamic Polarization (PDP), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray (EDX) analysis, quantum chemical calculations and molecular dynamics (MD) simulation. Results showed that inhibition effectiveness of MCA and MeCA increases with their concentration. Polarization results showed that MCA and MeCA displayed mixed type behaviour. Inhibition efficiencies of MCA and MeCA followed the order: 88% (MCA) < 74% (MeCA). MeCA and MCA inhibit corrosion by adsorbing on MS surface and their adsorption mode followed Langmuir adsorption isotherm via physico-chemical adsorption. SEM/EDX observations of the electrode surface confirm the formation of a film on the metal surface. Theoretical calculations obtained from DFT and Molecular Dynamics (MD) simulation were used to investigate the correlation between the molecular structure of inhibitors and their corrosion inhibition performances, which agree well with the experimental results.
中文翻译:

1.0 M HCl 中低碳钢醛衍生物的详细 DFT/MD 模拟、表面分析和电化学计算机探索
低碳钢 (MS) 的防腐蚀是该行业的一个主要问题。制定有效的保护策略是一个受欢迎的研究领域。在这项工作中,评估了甲氧基肉桂醛(MCA)和甲基肉桂醛(MeCA)两种分子作为低碳钢在 1.0 M HCl 溶液中的缓蚀剂,为在腐蚀领域开发新型缓蚀剂平台提供了有力的基础。MCA 和 MeCA 的缓蚀性能已通过失重、阻抗谱 (EIS)、动电位极化 (PDP)、扫描电子显微镜 (SEM)、能量色散 X 射线 (EDX) 分析、量子化学计算和分子动力学 (MD) 模拟。结果表明,MCA和MeCA的抑制效果随浓度增加而增加。极化结果表明 MCA 和 MeCA 表现出混合型行为。MCA 和 MeCA 的抑制效率依次为:88% (MCA) < 74% (MeCA)。MeCA和MCA通过吸附在MS表面上来抑制腐蚀,它们的吸附模式通过物理化学吸附遵循Langmuir吸附等温线。电极表面的 SEM/EDX 观察证实了在金属表面形成了一层薄膜。通过DFT和分子动力学(MD)模拟得到的理论计算,研究了缓蚀剂分子结构与其缓蚀性能之间的相关性,与实验结果吻合较好。MeCA和MCA通过吸附在MS表面上来抑制腐蚀,它们的吸附模式通过物理化学吸附遵循Langmuir吸附等温线。电极表面的 SEM/EDX 观察证实了在金属表面形成了一层薄膜。通过DFT和分子动力学(MD)模拟得到的理论计算,研究了缓蚀剂分子结构与其缓蚀性能之间的相关性,与实验结果吻合较好。MeCA和MCA通过吸附在MS表面上来抑制腐蚀,它们的吸附模式通过物理化学吸附遵循Langmuir吸附等温线。电极表面的 SEM/EDX 观察证实了在金属表面形成了一层薄膜。通过DFT和分子动力学(MD)模拟得到的理论计算,研究了缓蚀剂分子结构与其缓蚀性能之间的相关性,与实验结果吻合较好。

































 京公网安备 11010802027423号
京公网安备 11010802027423号