当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Anionic Redox and Suppressed Structural Disordering Enabling High-Energy and Long-Life Li-Rich Layered-Oxide Cathode
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-10-28 , DOI: 10.1002/aenm.202102311 Jinho Ahn 1 , Jungmin Kang 1 , Min‐kyung Cho 2 , Hyunyoung Park 1 , Wonseok Ko 1 , Yongseok Lee 1 , Hyun‐Soo Kim 3 , Young Hwa Jung 4 , Tae‐Yeol Jeon 4 , Hyungsub Kim 5 , Won‐Hee Ryu 3 , Jihyun Hong 6 , Jongsoon Kim 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2021-10-28 , DOI: 10.1002/aenm.202102311 Jinho Ahn 1 , Jungmin Kang 1 , Min‐kyung Cho 2 , Hyunyoung Park 1 , Wonseok Ko 1 , Yongseok Lee 1 , Hyun‐Soo Kim 3 , Young Hwa Jung 4 , Tae‐Yeol Jeon 4 , Hyungsub Kim 5 , Won‐Hee Ryu 3 , Jihyun Hong 6 , Jongsoon Kim 1
Affiliation
|
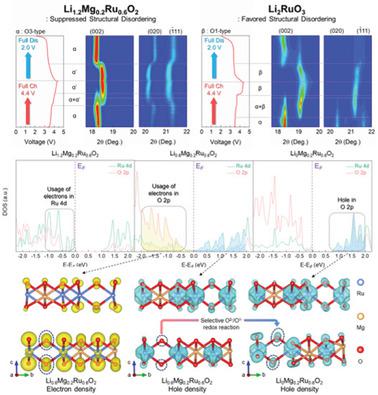
Despite their high energy densities, Li-rich layered oxides suffer from low capacity retention and continuous voltage decay caused by the migration of transition-metal cations into the Li layers. The cation migration stabilizes oxidized oxygen anions through the decoordination of oxygen from the metal once the anions participate in the redox reaction. Structural disordering is thus considered inevitable in most Li-rich layered oxides. However, herein, a Mg-substituted Li-rich layered oxide, Li1.2Mg0.2Ru0.6O2, with high structural and electrochemical stability is presented. Although using both cationic and anionic redox reactions, Ru migration in Li1.2−xMg0.2Ru0.6O2 is thermodynamically unfavored as a result of selectively oxidized O ions, suppressed structural disordering, and the formation of short (1.75 Å) Ru=O bonds enabled within the layered framework, which effectively decoordinate the oxidized O ions. The unprecedentedly high structural stability of Li1.2Mg0.2Ru0.6O2 leads to not only a high energy density of 964 Wh kg−1 but also outstanding rate capability and cycle performance. These findings demonstrate the potential of this practical strategy for the stabilization of Li-rich layered oxides even with prolonged cycling.
中文翻译:

选择性阴离子氧化还原和抑制结构无序使高能量和长寿命富锂层状氧化物阴极成为可能
尽管它们的能量密度高,但富锂层状氧化物由于过渡金属阳离子迁移到锂层而导致容量保持率低和电压持续衰减。一旦阴离子参与氧化还原反应,阳离子迁移就会通过金属中氧的脱位来稳定氧化的氧阴离子。因此,在大多数富锂层状氧化物中,结构无序被认为是不可避免的。然而,本文提出了一种具有高结构和电化学稳定性的镁取代的富锂层状氧化物 Li 1.2 Mg 0.2 Ru 0.6 O 2。尽管同时使用阳离子和阴离子氧化还原反应,Ru 在 Li 1.2− x Mg 0.2 Ru 中的迁移由于选择性氧化 O 离子、抑制结构无序以及在层状框架内形成短 (1.75 Å) Ru=O 键,0.6 O 2在热力学上是不利的,这有效地使氧化的 O 离子脱位。Li 1.2 Mg 0.2 Ru 0.6 O 2前所未有的高结构稳定性不仅导致964 Wh kg -1的高能量密度,而且还具有出色的倍率性能和循环性能。这些发现证明了即使在长时间循环的情况下,这种实用策略也具有稳定富锂层状氧化物的潜力。
更新日期:2021-12-16
中文翻译:

选择性阴离子氧化还原和抑制结构无序使高能量和长寿命富锂层状氧化物阴极成为可能
尽管它们的能量密度高,但富锂层状氧化物由于过渡金属阳离子迁移到锂层而导致容量保持率低和电压持续衰减。一旦阴离子参与氧化还原反应,阳离子迁移就会通过金属中氧的脱位来稳定氧化的氧阴离子。因此,在大多数富锂层状氧化物中,结构无序被认为是不可避免的。然而,本文提出了一种具有高结构和电化学稳定性的镁取代的富锂层状氧化物 Li 1.2 Mg 0.2 Ru 0.6 O 2。尽管同时使用阳离子和阴离子氧化还原反应,Ru 在 Li 1.2− x Mg 0.2 Ru 中的迁移由于选择性氧化 O 离子、抑制结构无序以及在层状框架内形成短 (1.75 Å) Ru=O 键,0.6 O 2在热力学上是不利的,这有效地使氧化的 O 离子脱位。Li 1.2 Mg 0.2 Ru 0.6 O 2前所未有的高结构稳定性不仅导致964 Wh kg -1的高能量密度,而且还具有出色的倍率性能和循环性能。这些发现证明了即使在长时间循环的情况下,这种实用策略也具有稳定富锂层状氧化物的潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号