当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azo(xy) vs Aniline Selectivity in Catalytic Nitroarene Reduction by Intermetallics: Experiments and Simulations
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-10-27 , DOI: 10.1021/acs.jpcc.1c08569 Carena L. Daniels 1 , Da-Jiang Liu 2 , Marquix A. S. Adamson 1 , Megan Knobeloch 1 , Javier Vela 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-10-27 , DOI: 10.1021/acs.jpcc.1c08569 Carena L. Daniels 1 , Da-Jiang Liu 2 , Marquix A. S. Adamson 1 , Megan Knobeloch 1 , Javier Vela 1, 2
Affiliation
|
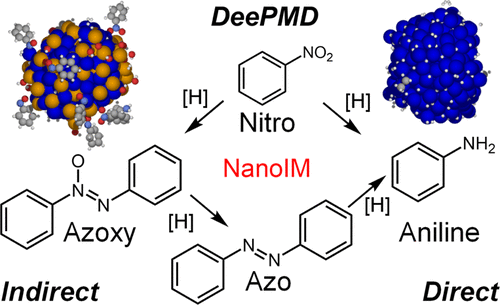
Intermetallic nanoparticles are promising catalysts in hydrogenation and fuel cell technologies. Much is known about the ability of intermetallic nanoparticles to selectively reduce nitro vs alkene, alcohol, or halide functional groups; less is known about their selectivity toward aniline vs azo or azoxy condensation products that result from the reduction of a nitro group alone. Because azo(xy)arenes bear promise as dyes, chemical stabilizers, and building blocks to functional materials but can be difficult to isolate, developing high surface area nanoparticle catalysts that display azo(xy) selectivity is desirable. To address this question, we studied a family of nanocrystalline group 10 metal (Pd, Pt)- and group 14 metal (Ge, Sn, Pb)-containing intermetallics─Pd2Ge, Pd2Sn, Pd3Sn2, Pd3Pb, and PtSn─in the catalytic reduction of nitroarenes. In contrast to monometallic Au, Pt, and Pd nanoparticles and ″random″ PdxSn1 – x nanoalloys, which are selective for aniline, nanoparticles of atomically precise intermetallic Pd2Ge, Pd2Sn, Pd3Sn2, and PtSn prefer an indirect condensation pathway and have a high selectivity for the azo(xy) products. The only exception is Pd3Pb, the most active among the intermetallic nanoparticles studied here, which is instead selective for aniline. Employing a novel application of molecular dynamics─based on machine learned potentials within a DeePMD framework─to heterogeneous catalysis, we are able to identify key reaction species on the different types of catalysts employed, furthering our understanding of the unique selectivity of these materials. By demonstrating how intermetallic nanoparticles can be as active yet more selective than other more traditional catalysts, this work provides new physical insights and opens new opportunities in the use of these materials in other important chemical transformations and applications.
中文翻译:

金属间化合物催化硝基芳烃还原中偶氮(xy)与苯胺的选择性:实验和模拟
金属间化合物纳米粒子是加氢和燃料电池技术中很有前景的催化剂。关于金属间化合物纳米粒子选择性还原硝基与烯烃、醇或卤化物官能团的能力,我们已经了解很多;关于它们对苯胺与仅由硝基还原产生的偶氮或偶氮氧缩合产物的选择性知之甚少。由于偶氮(xy)芳烃有望作为染料、化学稳定剂和功能材料的构建块,但难以分离,因此需要开发具有偶氮(xy)选择性的高表面积纳米颗粒催化剂。为了解决这个问题,我们研究了一系列含有第 10 族金属(Pd、Pt)和第 14 族金属(Ge、Sn、Pb)的金属间化合物─Pd 2Ge、Pd 2 Sn、Pd 3 Sn 2、Pd 3 Pb 和 PtSn─在硝基芳烃的催化还原中。与对苯胺有选择性的单金属 Au、Pt 和 Pd 纳米粒子和“随机”Pd x Sn 1 – x纳米合金相比,原子级精确的金属间化合物 Pd 2 Ge、Pd 2 Sn、Pd 3 Sn 2和 PtSn 的纳米粒子更受欢迎间接缩合途径,对偶氮(xy)产物具有高选择性。唯一的例外是 Pd 3Pb 是这里研究的金属间化合物纳米粒子中最活跃的,它对苯胺具有选择性。利用分子动力学的新应用——基于 DeePMD 框架内的机器学习潜力——多相催化,我们能够识别所用不同类型催化剂的关键反应物种,进一步了解这些材料的独特选择性。通过展示金属间纳米粒子如何与其他更传统的催化剂一样具有活性但更具选择性,这项工作提供了新的物理见解,并为这些材料在其他重要化学转化和应用中的使用开辟了新的机会。
更新日期:2021-11-11
中文翻译:

金属间化合物催化硝基芳烃还原中偶氮(xy)与苯胺的选择性:实验和模拟
金属间化合物纳米粒子是加氢和燃料电池技术中很有前景的催化剂。关于金属间化合物纳米粒子选择性还原硝基与烯烃、醇或卤化物官能团的能力,我们已经了解很多;关于它们对苯胺与仅由硝基还原产生的偶氮或偶氮氧缩合产物的选择性知之甚少。由于偶氮(xy)芳烃有望作为染料、化学稳定剂和功能材料的构建块,但难以分离,因此需要开发具有偶氮(xy)选择性的高表面积纳米颗粒催化剂。为了解决这个问题,我们研究了一系列含有第 10 族金属(Pd、Pt)和第 14 族金属(Ge、Sn、Pb)的金属间化合物─Pd 2Ge、Pd 2 Sn、Pd 3 Sn 2、Pd 3 Pb 和 PtSn─在硝基芳烃的催化还原中。与对苯胺有选择性的单金属 Au、Pt 和 Pd 纳米粒子和“随机”Pd x Sn 1 – x纳米合金相比,原子级精确的金属间化合物 Pd 2 Ge、Pd 2 Sn、Pd 3 Sn 2和 PtSn 的纳米粒子更受欢迎间接缩合途径,对偶氮(xy)产物具有高选择性。唯一的例外是 Pd 3Pb 是这里研究的金属间化合物纳米粒子中最活跃的,它对苯胺具有选择性。利用分子动力学的新应用——基于 DeePMD 框架内的机器学习潜力——多相催化,我们能够识别所用不同类型催化剂的关键反应物种,进一步了解这些材料的独特选择性。通过展示金属间纳米粒子如何与其他更传统的催化剂一样具有活性但更具选择性,这项工作提供了新的物理见解,并为这些材料在其他重要化学转化和应用中的使用开辟了新的机会。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号