Chemosphere ( IF 8.1 ) Pub Date : 2021-10-27 , DOI: 10.1016/j.chemosphere.2021.132729 Hongyan Zhai 1 , Jun Zhao 1 , Rumeng Wang 1 , Yuwei Yan 1 , Shanshan Yu 1 , Yingxin Zhao 1
|
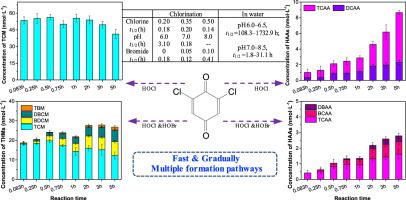
As a typical aromatic disinfection byproduct (DBP), 2,6-dichloro-1,4-benzoquinone (2,6-DCBQ) attracts much concern due to the potential toxicity. To further evaluate the role of 2,6-DCBQ as an intermediate DBP in water with or without chlorine, their decomposition characteristics and transformation potential to the regulated DBPs (i.e., trihalomethanes (THMs) and haloacetic acids (HAAs)) were investigated under different chlorine doses, pH values, temperatures, contact times, and bromide levels. The decomposition of 2,6-DCBQ under different conditions all fit apparent first-order kinetics. The hydrolysis rate constants of 2,6-DCBQ significantly increased with pH. The half-live values of 2,6-DCBQ were 108.3–1732.9 h at pH 6.0–6.5, and 1.8–31.1 h at pH 7.0–8.5. During the hydrolysis of 2,6-DCBQ, there was no THMs and HAAs generated. During chlorination, 2,6-DCBQ decayed rapidly accompanied by the fast formation of trichloromethane (TCM) and the gradual generation of dichloroacetic acid and trichloroacetic acid. The molar conversion rates of 2,6-DCBQ-to-THMs (i.e., TCM) and 2,6-DCBQ-to-HAAs were 2.9–10.0% and 0.1–2.2% under vary conditions. The presence of bromide increased the conversion rates of 2,6-DCBQ-to-THMs and caused the generation of brominated THMs and HAAs. According to the decomposition characteristics of 2,6-DCBQ and the formation trends of THMs and HAAs under different conditions, multiple formation pathways from 2,6-DCBQ to THMs and HAAs were proposed.
中文翻译:

氯化过程中 2,6-二氯-1,4-苯醌形成三卤甲烷和卤乙酸:分解动力学、转化率和途径
作为典型的芳香族消毒副产物(DBP),2,6-二氯-1,4-苯醌(2,6-DCBQ)因其潜在的毒性而备受关注。为了进一步评估 2,6-DCBQ 在含氯或不含氯的水中作为中间体 DBP 的作用,研究了它们在不同条件下的分解特性和向受管制 DBP(即三卤甲烷 (THM) 和卤乙酸 (HAAs))的转化潜力。氯剂量、pH 值、温度、接触时间和溴化物水平。2,6-DCBQ在不同条件下的分解均符合表观一级动力学。2,6-DCBQ 的水解速率常数随 pH 值显着增加。2,6-DCBQ 的半衰期值在 pH 6.0-6.5 时为 108.3-1732.9 h,在 pH 7.0-8.5 时为 1.8-31.1 h。在 2,6-DCBQ 的水解过程中,没有产生 THMs 和 HAAs。在氯化过程中,2,6-DCBQ 迅速衰变,伴随着三氯甲烷 (TCM) 的快速形成以及二氯乙酸和三氯乙酸的逐渐生成。在不同条件下,2,6-DCBQ 到 THM(即 TCM)和 2,6-DCBQ 到 HAA 的摩尔转化率分别为 2.9-10.0% 和 0.1-2.2%。溴化物的存在提高了 2,6-DCBQ 到 THM 的转化率,并导致溴化 THM 和 HAA 的产生。根据2,6-DCBQ的分解特性以及不同条件下THMs和HAAs的形成趋势,提出了2,6-DCBQ到THMs和HAAs的多种形成途径。在不同条件下,6-DCBQ-to-THM(即 TCM)和 2,6-DCBQ-to-HAA 分别为 2.9-10.0% 和 0.1-2.2%。溴化物的存在提高了 2,6-DCBQ 到 THM 的转化率,并导致溴化 THM 和 HAA 的产生。根据2,6-DCBQ的分解特性以及不同条件下THMs和HAAs的形成趋势,提出了2,6-DCBQ到THMs和HAAs的多种形成途径。在不同条件下,6-DCBQ-to-THM(即 TCM)和 2,6-DCBQ-to-HAA 分别为 2.9-10.0% 和 0.1-2.2%。溴化物的存在提高了 2,6-DCBQ 到 THM 的转化率,并导致溴化 THM 和 HAA 的产生。根据2,6-DCBQ的分解特性以及不同条件下THMs和HAAs的形成趋势,提出了2,6-DCBQ到THMs和HAAs的多种形成途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号