当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetic CuNi Alloy Nanoparticles for Catalytic Transfer Hydrogenation of Nitroarene
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-10-27 , DOI: 10.1021/acs.iecr.1c03175 Hongbin Shi 1 , Xiaofeng Dai 1 , Qing Liu 1 , Teng Zhang 1 , Yabing Zhang 1 , Yuling Shi 1 , Tao Wang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-10-27 , DOI: 10.1021/acs.iecr.1c03175 Hongbin Shi 1 , Xiaofeng Dai 1 , Qing Liu 1 , Teng Zhang 1 , Yabing Zhang 1 , Yuling Shi 1 , Tao Wang 1
Affiliation
|
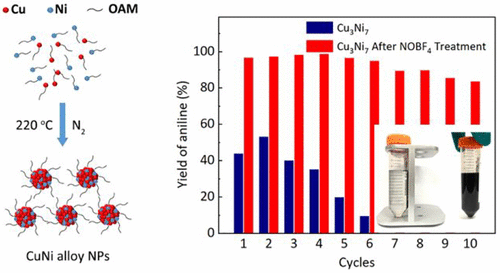
Magnetic CuNi alloy nanoparticles (CuNi NPs) for the catalytic transfer hydrogenation (CTH) of nitroarene were successfully prepared by the facile thermal decomposition of copper formate (Cuf) and nickel formate (Nif) simultaneously. Liquid paraffin served as solvent, and oleylamine (OAM) acted as both complexing agent and stabilizer. The particle size and Cu/Ni composition of CuNi NPs could be controlled by adjusting the amount of OAM and the Cu/Ni ratio of the precursor. The average diameter of the prepared CuNi NPs ranged from 10.2 to 56.9 nm. It had been confirmed that CuNi NPs were an alloy of Cu and Ni and effectively resistant to oxidation. The prepared CuNi NPs could be separated under an external magnetic field without any energy consumption since they were superparamagnetic. Among the prepared CuNi NPs with different compositions, the Cu3Ni7 NPs were the best catalyst for catalytic transfer hydrogenation of nitrobenzene with ethylene glycol (EG) as the hydrogen donor, giving a nitrobenzene conversion of 99% and an aniline yield of >97%. The conversion and yield remained above 95% and 80% respectively after 10 cycles of NOBF4-treated Cu3Ni7 NPs, demonstrating good reusability for the CTH reaction. This work reported a novel method for preparing magnetic CuNi NPs and also provided a new approach for the hydrogenation of nitroarene catalyzed by non-noble metals in a green environment.
中文翻译:

用于硝基芳烃催化转移加氢的磁性CuNi合金纳米颗粒
通过同时对甲酸铜 (Cuf) 和甲酸镍 (Nif) 进行简单的热分解,成功制备了用于硝基芳烃催化转移氢化 (CTH) 的磁性 CuNi 合金纳米粒子 (CuNi NPs)。液体石蜡作为溶剂,油胺(OAM)作为络合剂和稳定剂。通过调节 OAM 的量和前驱体的 Cu/Ni 比,可以控制 CuNi NPs 的粒径和 Cu/Ni 组成。制备的 CuNi NPs 的平均直径范围为 10.2 至 56.9 nm。已经证实CuNi NPs是Cu和Ni的合金并且有效地抗氧化。制备的 CuNi NPs 具有超顺磁性,因此可以在外部磁场下分离而不消耗任何能量。在制备的不同成分的 CuNi NPs 中,Cu3 Ni 7 NPs是以乙二醇(EG)为氢供体催化硝基苯催化转移加氢的最佳催化剂,硝基苯转化率为99%,苯胺产率>97%。在NOBF 4处理的Cu 3 Ni 7 NPs循环10 次后,转化率和产率分别保持在95% 和80% 以上,表明CTH 反应具有良好的可重复使用性。该工作报道了一种制备磁性CuNi NPs的新方法,也为绿色环境中非贵金属催化硝基芳烃的加氢提供了一种新方法。
更新日期:2021-11-10
中文翻译:

用于硝基芳烃催化转移加氢的磁性CuNi合金纳米颗粒
通过同时对甲酸铜 (Cuf) 和甲酸镍 (Nif) 进行简单的热分解,成功制备了用于硝基芳烃催化转移氢化 (CTH) 的磁性 CuNi 合金纳米粒子 (CuNi NPs)。液体石蜡作为溶剂,油胺(OAM)作为络合剂和稳定剂。通过调节 OAM 的量和前驱体的 Cu/Ni 比,可以控制 CuNi NPs 的粒径和 Cu/Ni 组成。制备的 CuNi NPs 的平均直径范围为 10.2 至 56.9 nm。已经证实CuNi NPs是Cu和Ni的合金并且有效地抗氧化。制备的 CuNi NPs 具有超顺磁性,因此可以在外部磁场下分离而不消耗任何能量。在制备的不同成分的 CuNi NPs 中,Cu3 Ni 7 NPs是以乙二醇(EG)为氢供体催化硝基苯催化转移加氢的最佳催化剂,硝基苯转化率为99%,苯胺产率>97%。在NOBF 4处理的Cu 3 Ni 7 NPs循环10 次后,转化率和产率分别保持在95% 和80% 以上,表明CTH 反应具有良好的可重复使用性。该工作报道了一种制备磁性CuNi NPs的新方法,也为绿色环境中非贵金属催化硝基芳烃的加氢提供了一种新方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号