当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Thiocyanation and Aromatic Amination To Achieve Organized Annulation of Enaminone with Thiocyanate
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-25 , DOI: 10.1021/acs.orglett.1c03129 Jun Zhang 1 , Mengyao She 1, 2 , Lang Liu 1 , Xukai Feng 1 , Yao Li 1 , Hua Liu 1 , Tingting Zheng 1 , Xin Leng 1 , Ping Liu 1 , Shengyong Zhang 1 , Jianli Li 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-25 , DOI: 10.1021/acs.orglett.1c03129 Jun Zhang 1 , Mengyao She 1, 2 , Lang Liu 1 , Xukai Feng 1 , Yao Li 1 , Hua Liu 1 , Tingting Zheng 1 , Xin Leng 1 , Ping Liu 1 , Shengyong Zhang 1 , Jianli Li 1
Affiliation
|
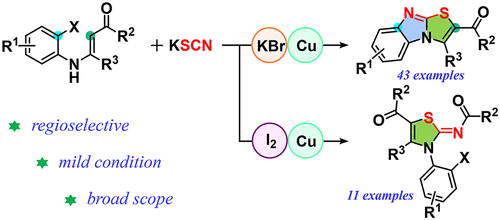
A tandem insertion of thiocyanate to enamine was performed for the regioselective synthesis of multisubstituted benzoimidazo[2,1-b]thiazoles. This method was shown to be effective in addressing the issue of isomerization encountered in common strategies. With a change made to the leading group on the aniline fragment of enamine, the reaction achieved different transformations, thus enabling multisubstituted benzo[4,5]imidazo[2,1-b]thiazoles and thiazoles in satisfactory yields.
中文翻译:

选择性硫氰化和芳香胺化以实现烯胺酮与硫氰酸酯的有序环化
硫氰酸盐与烯胺的串联插入用于区域选择性合成多取代苯并咪唑并[2,1- b ]噻唑。该方法被证明可有效解决常见策略中遇到的异构化问题。通过改变烯胺苯胺片段上的前导基团,该反应实现了不同的转化,从而以令人满意的收率获得了多取代的苯并[4,5]咪唑并[2,1- b ]噻唑和噻唑。
更新日期:2021-11-05
中文翻译:

选择性硫氰化和芳香胺化以实现烯胺酮与硫氰酸酯的有序环化
硫氰酸盐与烯胺的串联插入用于区域选择性合成多取代苯并咪唑并[2,1- b ]噻唑。该方法被证明可有效解决常见策略中遇到的异构化问题。通过改变烯胺苯胺片段上的前导基团,该反应实现了不同的转化,从而以令人满意的收率获得了多取代的苯并[4,5]咪唑并[2,1- b ]噻唑和噻唑。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号