当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodivergent Synthesis of Both Z- and E-Alkenes by Photoinduced, Ni-Catalyzed Enantioselective C(sp3)–H Alkenylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-25 , DOI: 10.1021/acscatal.1c04314 Jitao Xu 1 , Zhilong Li 1 , Yumin Xu 1 , Xiaomin Shu 1 , Haohua Huo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-10-25 , DOI: 10.1021/acscatal.1c04314 Jitao Xu 1 , Zhilong Li 1 , Yumin Xu 1 , Xiaomin Shu 1 , Haohua Huo 1
Affiliation
|
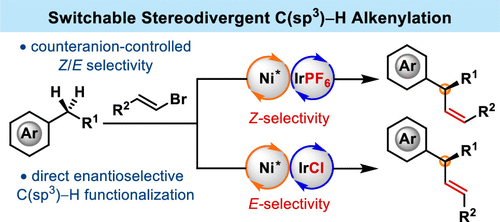
The enantio- and stereoselective synthesis of stereodefined alkenes, especially the functionalized Z-isomer with an allylic stereogenic center, remains a great challenge. We herein report an enantioselective benzylic C(sp3)–H alkenylation of simple alkylarenes with vinyl bromides via photoinduced nickel catalysis, which allows for the stereodivergent synthesis of both enantioenriched Z- and E-alkenes bearing aryl-substituted, allylic tertiary stereogenic centers. Interestingly, the tunable Z/E-selectivity is achieved by energy transfer catalysis via a judicious choice of the photocatalyst counteranion. This versatile strategy features simple starting materials, mild reaction conditions, broad substrate scope, divergent Z- and E-selectivity, and high enantioselectivities. Moreover, a formal asymmetric benzylic C(sp3)–H alkylation can also be achieved via a one-pot alkenylation/reduction sequence, providing a complementary strategy to address the notoriously challenging stereochemical control in C(sp3)–C(sp3) bond construction.
中文翻译:

通过光诱导、镍催化的对映选择性 C(sp3)-H 烯基化立体发散合成 Z-和 E-烯烃
立体定义烯烃的对映选择性和立体选择性合成,尤其是具有烯丙基立体中心的功能化Z异构体,仍然是一个巨大的挑战。我们在此报告了通过光诱导镍催化对简单烷基芳烃与乙烯基溴的对映选择性苄基 C(sp 3 )-H 烯基化,这允许对映体富集的Z - 和E -烯烃的立体发散合成,这些烯烃带有芳基取代的烯丙基叔立体中心。有趣的是,可调Z / E-选择性是通过光催化剂反阴离子的明智选择通过能量转移催化实现的。这种通用策略具有简单的起始材料、温和的反应条件、广泛的底物范围、不同的Z - 和E -选择性以及高对映选择性。此外,形式上的不对称苄基 C(sp 3 )–H 烷基化也可以通过一锅烯基化/还原序列实现,为解决 C(sp 3 )–C(sp 3 ) 中臭名昭著的立体化学控制提供了补充策略) 债券建设。
更新日期:2021-11-05
中文翻译:

通过光诱导、镍催化的对映选择性 C(sp3)-H 烯基化立体发散合成 Z-和 E-烯烃
立体定义烯烃的对映选择性和立体选择性合成,尤其是具有烯丙基立体中心的功能化Z异构体,仍然是一个巨大的挑战。我们在此报告了通过光诱导镍催化对简单烷基芳烃与乙烯基溴的对映选择性苄基 C(sp 3 )-H 烯基化,这允许对映体富集的Z - 和E -烯烃的立体发散合成,这些烯烃带有芳基取代的烯丙基叔立体中心。有趣的是,可调Z / E-选择性是通过光催化剂反阴离子的明智选择通过能量转移催化实现的。这种通用策略具有简单的起始材料、温和的反应条件、广泛的底物范围、不同的Z - 和E -选择性以及高对映选择性。此外,形式上的不对称苄基 C(sp 3 )–H 烷基化也可以通过一锅烯基化/还原序列实现,为解决 C(sp 3 )–C(sp 3 ) 中臭名昭著的立体化学控制提供了补充策略) 债券建设。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号