当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Rationale for Selectivity in the Asymmetric Allylic Alkylation of Cycloalkenyl Esters Employing the Trost ‘Standard Ligand’ (TSL): Isolation, Analysis and Alkylation of the Monomeric form of the Cationic η3-Cyclohexenyl Complex [(η3-c-C6H9)Pd(TSL)]+
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-07-29 , DOI: 10.1021/ja8099757 Craig P. Butts 1 , Emane Filali 1 , Guy C. Lloyd-Jones 1 , Per-Ola Norrby 1 , David A. Sale 1 , York Schramm 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-07-29 , DOI: 10.1021/ja8099757 Craig P. Butts 1 , Emane Filali 1 , Guy C. Lloyd-Jones 1 , Per-Ola Norrby 1 , David A. Sale 1 , York Schramm 1
Affiliation
|
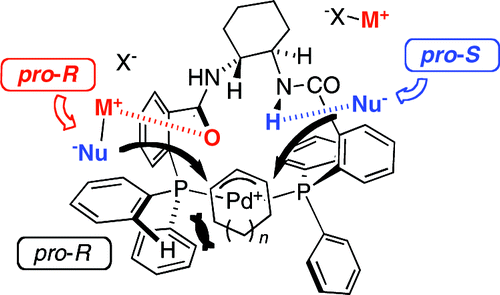
The solution-phase structures of the monomeric forms of the cationic Pd-eta(3)-allyl and Pd-eta(3)-cyclohexenyl complexes [Pd(R,R)-1(eta(3)-C(3)H(5))](+) (7(+)) and [Pd(R,R)-1(eta(3)-C(6)H(9))](+) (8(+)) bearing the trans-cyclohexylenediamine-based Trost 'Standard Ligand' (R,R)-1 have been elucidated by NMR, isotopic labeling and computation. In both complexes, (R,R)-1 is found to adopt a C(1)-symmetric conformation, leading to a concave shape in the 13-membered chelate in which one amide group in the chiral scaffold projects its NH unit out of the concave surface in close vicinity to one allyl terminus. The adjacent amide has a reversed orientation and projects its carbonyl group out of the concave face in the vicinity of the opposite allyl terminus. Stoichiometric and catalytic asymmetric alkylations of [8(+)][X(-)] by MCHE(2) (E = ester, M = 'escort' counterion, X = Pd allyl counterion) show the same selectivities and trends as have been reported for in situ-generated catalysts, and a new model for the enantioselectivity has been explored computationally. Three factors are found to govern the regioselectivity (pro-S vs pro-R) of attack of nucleophiles on the eta(3)-C(6)H(9) ring in 8(+) and thus the ee of the alkylation product: (i) a pro-R torquoselective bias is induced by steric interaction of the eta(3)-C(6)H(9) moiety with one phenyl ring of the ligand; (ii) pro-S delivery of the nucleophile can be facilitated by hydrogen-bonding with the concave orientated amide N-H; and (iii) pro-R delivery of the nucleophile can be facilitated by escort ion (M) binding to the concave orientated amide carbonyl. The latter two opposing interactions lead to the selectivity of the alkylation being sensitive to the identities of X(-) and M(+). The generation of 8(+) from cyclohexenyl ester substrate has also been explored computationally. The concave orientated amide N-H is able to activate the leaving group of the allylic ester by hydrogen bonding to its carbonyl group. However, this interaction is only feasible for the (S)-enantiomer of substrate, leading to the prediction of a powerful kinetic resolution (k(S) >> k(R)), as is found experimentally. This new model involving two regiochemically distinct (NH) and (CO) locations for nucleofuge or nucleophile binding, may prove of broad utility for the interpretation of the selectivity in asymmetric allylic alkylation reactions catalyzed by Pd complexes of (R,R)-1 and related ligands.
中文翻译:

采用 Trost '标准配体' (TSL) 的环烯基酯的不对称烯丙基烷基化选择性的基于结构的基本原理:阳离子 η3-环己烯基复合物 [(η3-c-C6H9)Pd 单体形式的分离、分析和烷基化(TSL)]+
阳离子 Pd-eta(3)-烯丙基和 Pd-eta(3)-环己烯基配合物 [Pd(R,R)-1(eta(3)-C(3)H) 单体形式的溶液相结构(5))](+) (7(+)) 和 [Pd(R,R)-1(eta(3)-C(6)H(9))](+) (8(+)) 轴承已通过核磁共振、同位素标记和计算阐明了基于反式环己二胺的 Trost '标准配体' (R,R)-1。在这两种复合物中,(R,R)-1 被发现采用 C(1) 对称构象,导致 13 元螯合物中的凹形,其中手性支架中的一个酰胺基团将其 NH 单元伸出靠近一个烯丙基末端的凹面。相邻的酰胺具有相反的取向,并将其羰基突出到相对烯丙基末端附近的凹面之外。MCHE(2) 对 [8(+)][X(-)] 的化学计量和催化不对称烷基化(E = 酯,M = '护送'反离子,X = Pd 烯丙基反离子)显示出与原位生成催化剂相同的选择性和趋势,并且已经通过计算探索了对映选择性的新模型。发现三个因素控制亲核试剂攻击 8(+) 中的 eta(3)-C(6)H(9) 环的区域选择性(pro-S vs pro-R),从而控制烷基化产物的 ee : (i) eta(3)-C(6)H(9) 部分与配体的一个苯环的空间相互作用诱导了 pro-R 扭矩选择性偏差;(ii) 亲核试剂的 pro-S 传递可以通过与凹面取向的酰胺 NH 形成氢键来促进;和 (iii) 亲核试剂的 pro-R 传递可以通过护送离子 (M) 与凹取向的酰胺羰基结合来促进。后两种相反的相互作用导致烷基化的选择性对 X(-) 和 M(+) 的身份敏感。从环己烯基酯底物生成 8(+) 的过程也已在计算上进行了探索。凹取向的酰胺 NH 能够通过氢键与其羰基结合来激活烯丙酯的离去基团。然而,这种相互作用仅适用于底物的 (S)-对映异构体,导致预测强大的动力学分辨率 (k(S) >> k(R)),正如实验所发现的。这种新模型涉及两个区域化学上不同的 (NH) 和 (CO) 位置,用于离核或亲核试剂结合,可能证明在解释由 (R,R)-1 和相关配体。
更新日期:2009-07-29
中文翻译:

采用 Trost '标准配体' (TSL) 的环烯基酯的不对称烯丙基烷基化选择性的基于结构的基本原理:阳离子 η3-环己烯基复合物 [(η3-c-C6H9)Pd 单体形式的分离、分析和烷基化(TSL)]+
阳离子 Pd-eta(3)-烯丙基和 Pd-eta(3)-环己烯基配合物 [Pd(R,R)-1(eta(3)-C(3)H) 单体形式的溶液相结构(5))](+) (7(+)) 和 [Pd(R,R)-1(eta(3)-C(6)H(9))](+) (8(+)) 轴承已通过核磁共振、同位素标记和计算阐明了基于反式环己二胺的 Trost '标准配体' (R,R)-1。在这两种复合物中,(R,R)-1 被发现采用 C(1) 对称构象,导致 13 元螯合物中的凹形,其中手性支架中的一个酰胺基团将其 NH 单元伸出靠近一个烯丙基末端的凹面。相邻的酰胺具有相反的取向,并将其羰基突出到相对烯丙基末端附近的凹面之外。MCHE(2) 对 [8(+)][X(-)] 的化学计量和催化不对称烷基化(E = 酯,M = '护送'反离子,X = Pd 烯丙基反离子)显示出与原位生成催化剂相同的选择性和趋势,并且已经通过计算探索了对映选择性的新模型。发现三个因素控制亲核试剂攻击 8(+) 中的 eta(3)-C(6)H(9) 环的区域选择性(pro-S vs pro-R),从而控制烷基化产物的 ee : (i) eta(3)-C(6)H(9) 部分与配体的一个苯环的空间相互作用诱导了 pro-R 扭矩选择性偏差;(ii) 亲核试剂的 pro-S 传递可以通过与凹面取向的酰胺 NH 形成氢键来促进;和 (iii) 亲核试剂的 pro-R 传递可以通过护送离子 (M) 与凹取向的酰胺羰基结合来促进。后两种相反的相互作用导致烷基化的选择性对 X(-) 和 M(+) 的身份敏感。从环己烯基酯底物生成 8(+) 的过程也已在计算上进行了探索。凹取向的酰胺 NH 能够通过氢键与其羰基结合来激活烯丙酯的离去基团。然而,这种相互作用仅适用于底物的 (S)-对映异构体,导致预测强大的动力学分辨率 (k(S) >> k(R)),正如实验所发现的。这种新模型涉及两个区域化学上不同的 (NH) 和 (CO) 位置,用于离核或亲核试剂结合,可能证明在解释由 (R,R)-1 和相关配体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号