当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand-Enabled, Copper-Catalyzed Electrophilic Amination for the Asymmetric Synthesis of β-Amino Acids
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-23 , DOI: 10.1021/acs.orglett.1c03328 Raj K Tak 1 , Hidetoshi Noda 1 , Masakatsu Shibasaki 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-23 , DOI: 10.1021/acs.orglett.1c03328 Raj K Tak 1 , Hidetoshi Noda 1 , Masakatsu Shibasaki 1
Affiliation
|
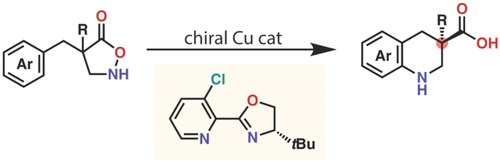
Catalytic asymmetric nitrene transfer has emerged as a reliable method for the synthesis of nitrogen-containing chiral compounds. Herein, we report the copper-catalyzed intramolecular asymmetric electrophilic amination of aromatic rings. The reactive intermediate is a copper-alkyl nitrene generated from isoxazolidin-5-ones. Copper catalysis promotes three classes of asymmetric transformations, namely, asymmetric desymmetrization, parallel kinetic resolution, and kinetic resolution, expanding the repertoire of alkyl nitrene transfer and providing various cyclic and linear β-amino acids in their enantioenriched forms.
中文翻译:

配体铜催化的亲电胺化用于 β-氨基酸的不对称合成
催化不对称氮烯转移已成为合成含氮手性化合物的可靠方法。在此,我们报道了铜催化的芳环分子内不对称亲电胺化。反应中间体是由 5-异恶唑烷酮生成的铜-烷基氮烯。铜催化促进三类不对称转化,即不对称去对称化、平行动力学拆分和动力学拆分,扩大了烷基氮烯转移的范围并提供对映体富集形式的各种环状和线性β-氨基酸。
更新日期:2021-11-05
中文翻译:

配体铜催化的亲电胺化用于 β-氨基酸的不对称合成
催化不对称氮烯转移已成为合成含氮手性化合物的可靠方法。在此,我们报道了铜催化的芳环分子内不对称亲电胺化。反应中间体是由 5-异恶唑烷酮生成的铜-烷基氮烯。铜催化促进三类不对称转化,即不对称去对称化、平行动力学拆分和动力学拆分,扩大了烷基氮烯转移的范围并提供对映体富集形式的各种环状和线性β-氨基酸。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号