当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Bioorthogonal-Activated Fluorescence Turn-On Probe Based on Nitrone-Modified 1,8-Naphthalimide for Live-Cell Imaging
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-10-21 , DOI: 10.1002/cjoc.202100563 Yu Teng 1 , Hong Yang 1 , Xiang Li 1 , Yongcheng Wang 1 , Dali Yin 1 , Yulin Tian 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2021-10-21 , DOI: 10.1002/cjoc.202100563 Yu Teng 1 , Hong Yang 1 , Xiang Li 1 , Yongcheng Wang 1 , Dali Yin 1 , Yulin Tian 1
Affiliation
|
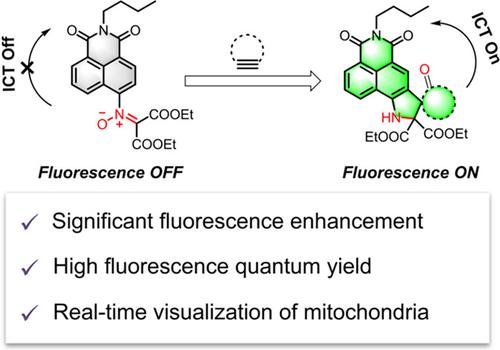
A nitrone-modified 1,8-naphthalimide was designed as a novel bioorthogonal-activated turn-on probe based on strain-promoted alkyne-nitrone cycloaddition (SPANC). The bioorthogonal cycloadducts were subsequently transformed into fluorescent rearrangement products by photo-acceleration, which exhibited significant fluorescence enhancement, large stokes shift, and high fluorescence quantum yield. DFT calculations were performed to elucidate the fluorescence OFF-ON mechanism. This fluorogenic strategy was successfully applied to labeling of proteins and visualizing mitochondria in live cells in real time.
中文翻译:

基于硝酮修饰的 1,8-萘二甲酰亚胺的生物正交激活荧光开启探针,用于活细胞成像
硝酮修饰的 1,8-萘二甲酰亚胺被设计为一种基于应变促进的炔-硝酮环加成 (SPANC) 的新型生物正交激活开启探针。随后通过光加速将生物正交环加合物转化为荧光重排产物,其表现出显着的荧光增强、大斯托克斯位移和高荧光量子产率。进行 DFT 计算以阐明荧光 OFF-ON 机制。这种荧光策略已成功应用于标记蛋白质和实时可视化活细胞中的线粒体。
更新日期:2021-12-13

中文翻译:

基于硝酮修饰的 1,8-萘二甲酰亚胺的生物正交激活荧光开启探针,用于活细胞成像
硝酮修饰的 1,8-萘二甲酰亚胺被设计为一种基于应变促进的炔-硝酮环加成 (SPANC) 的新型生物正交激活开启探针。随后通过光加速将生物正交环加合物转化为荧光重排产物,其表现出显着的荧光增强、大斯托克斯位移和高荧光量子产率。进行 DFT 计算以阐明荧光 OFF-ON 机制。这种荧光策略已成功应用于标记蛋白质和实时可视化活细胞中的线粒体。
































 京公网安备 11010802027423号
京公网安备 11010802027423号