当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 1-Amino-1H-imidazole-5-carboxamide Derivatives as Highly Selective, Covalent Bruton’s Tyrosine Kinase (BTK) Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-10-21 , DOI: 10.1021/acs.jmedchem.1c01559 Chunhua Ma 1 , Qingyun Li 1 , Minghao Zhao 1 , Goujie Fan 1 , Jie Zhao 1 , Dandan Zhang 1 , Shouning Yang 1 , Shuting Zhang 1 , Dingding Gao 2 , Longfei Mao 1, 3 , Liang Zhu 1 , Wei Li 1, 3 , Guiqing Xu 1 , Yuqin Jiang 1 , Qingjie Ding 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-10-21 , DOI: 10.1021/acs.jmedchem.1c01559 Chunhua Ma 1 , Qingyun Li 1 , Minghao Zhao 1 , Goujie Fan 1 , Jie Zhao 1 , Dandan Zhang 1 , Shouning Yang 1 , Shuting Zhang 1 , Dingding Gao 2 , Longfei Mao 1, 3 , Liang Zhu 1 , Wei Li 1, 3 , Guiqing Xu 1 , Yuqin Jiang 1 , Qingjie Ding 1
Affiliation
|
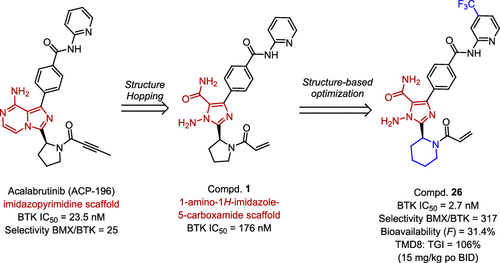
Bruton’s tyrosine kinase (BTK) inhibitors suppressing the aberrant activation of BTK have led to a paradigm shift in the therapy of B-cell malignancies. However, there is an urgent need to discover more selective covalent BTK inhibitors owing to the off-target adverse effects of the approved inhibitor, ibrutinib. Herein, we disclose the discovery and preliminary activity studies of novel BTK inhibitors carrying 1-amino-1H-imidazole-5-carboxamide as a hinge binder. The most potent BTK inhibitor 26 demonstrates impressive selectivity, favorable pharmacokinetic properties, and robust antitumor efficacy in vivo, which indicates its potential as a novel therapeutic option for B-cell lymphomas. Importantly, to the best of our knowledge, this is the first example of a 1-amino-1H-imidazole-5-carboxamide scaffold used as the hinge binder of kinase inhibitors, which will largely expand the chemical diversity of kinase inhibitors.
中文翻译:

发现 1-氨基-1H-咪唑-5-甲酰胺衍生物作为高选择性共价布鲁顿酪氨酸激酶 (BTK) 抑制剂
布鲁顿的酪氨酸激酶 (BTK) 抑制剂抑制 BTK 的异常激活导致 B 细胞恶性肿瘤治疗的范式转变。然而,由于批准的抑制剂依鲁替尼的脱靶副作用,迫切需要发现更具选择性的共价 BTK 抑制剂。在此,我们公开了携带 1-氨基-1 H-咪唑-5-甲酰胺作为铰链粘合剂的新型 BTK 抑制剂的发现和初步活性研究。最有效的 BTK 抑制剂26在体内表现出令人印象深刻的选择性、良好的药代动力学特性和强大的抗肿瘤功效,这表明其作为 B 细胞淋巴瘤的新治疗选择的潜力。重要的是,据我们所知,这是用作激酶抑制剂铰链粘合剂的1-氨基-1 H-咪唑-5-甲酰胺支架的第一个例子,这将在很大程度上扩大激酶抑制剂的化学多样性。
更新日期:2021-11-11
中文翻译:

发现 1-氨基-1H-咪唑-5-甲酰胺衍生物作为高选择性共价布鲁顿酪氨酸激酶 (BTK) 抑制剂
布鲁顿的酪氨酸激酶 (BTK) 抑制剂抑制 BTK 的异常激活导致 B 细胞恶性肿瘤治疗的范式转变。然而,由于批准的抑制剂依鲁替尼的脱靶副作用,迫切需要发现更具选择性的共价 BTK 抑制剂。在此,我们公开了携带 1-氨基-1 H-咪唑-5-甲酰胺作为铰链粘合剂的新型 BTK 抑制剂的发现和初步活性研究。最有效的 BTK 抑制剂26在体内表现出令人印象深刻的选择性、良好的药代动力学特性和强大的抗肿瘤功效,这表明其作为 B 细胞淋巴瘤的新治疗选择的潜力。重要的是,据我们所知,这是用作激酶抑制剂铰链粘合剂的1-氨基-1 H-咪唑-5-甲酰胺支架的第一个例子,这将在很大程度上扩大激酶抑制剂的化学多样性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号