当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theory and Experiment Demonstrate that Sb(V)-Promoted Methane C–H Activation and Functionalization Outcompete Superacid Protonolysis in Sulfuric Acid
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-10-19 , DOI: 10.1021/jacs.1c08170 Shu-Sen Chen 1 , Anjaneyulu Koppaka 2 , Roy A Periana 2 , Daniel H Ess 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-10-19 , DOI: 10.1021/jacs.1c08170 Shu-Sen Chen 1 , Anjaneyulu Koppaka 2 , Roy A Periana 2 , Daniel H Ess 1
Affiliation
|
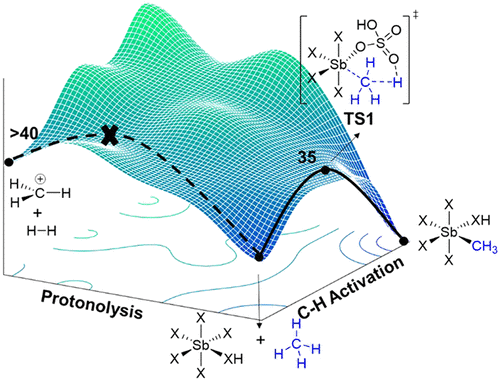
Sb(V) in strong Brønsted acid solvents is traditionally assumed to react with light alkanes through superacid protonolysis, which results in carbocation intermediates, H2, and carbon oligomerization. In contrast to this general assumption, our density functional theory (DFT) calculations revealed an accessible barrier for C–H activation between methane and Sb(V) in sulfuric acid that could potentially outcompete superacid protonolysis. This prompted us to experimentally examine this reaction in sulfuric acid with oleum, which has never been reported because of presumed superacid reactivity. Reaction of methane at 180 °C for 3 h resulted in very high yields of methyl bisulfate without significant overoxidation. Our DFT calculations show that a C–H activation and Sb-Me bond functionalization mechanism to give methyl bisulfate outcompetes methane protonolysis and many other possible reaction mechanisms, such as electron transfer, proton-coupled electron transfer, and hydride abstraction. Our DFT calculations also explain experimental hydrogen–deuterium exchange studies and the absence of methane carbo-functionalization/oligomerization products. Overall, this work demonstrates that in very strong Brønsted acid solvent, Sb(V) can induce innersphere reaction mechanisms akin to transition metals and outcompete superacid reactivity.
中文翻译:

理论和实验表明,Sb(V) 促进的甲烷 C-H 活化和功能化在硫酸中的超强酸质子分解中胜出
传统上认为,强布朗斯台德酸溶剂中的 Sb(V) 通过超酸质子分解与轻质烷烃反应,产生碳正离子中间体 H 2, 和碳齐聚。与这一一般假设相反,我们的密度泛函理论 (DFT) 计算揭示了硫酸中甲烷和 Sb(V) 之间 C-H 活化的可接近障碍,这可能会超过超酸质子分解。这促使我们通过实验检查硫酸与发烟硫酸中的这种反应,由于假定的超酸反应性而从未报道过。甲烷在 180 °C 下反应 3 小时导致非常高的硫酸氢甲酯产率,而没有明显的过氧化。我们的 DFT 计算表明,产生硫酸氢甲酯的 C-H 活化和 Sb-Me 键官能化机制胜过甲烷质子分解和许多其他可能的反应机制,例如电子转移、质子耦合电子转移和氢化物提取。我们的 DFT 计算还解释了实验性氢-氘交换研究以及不存在甲烷碳官能化/低聚产物。总体而言,这项工作表明,在非常强的布朗斯台德酸溶剂中,Sb(V) 可以诱导类似于过渡金属的内层反应机制,并超过超强酸的反应性。
更新日期:2021-11-03
中文翻译:

理论和实验表明,Sb(V) 促进的甲烷 C-H 活化和功能化在硫酸中的超强酸质子分解中胜出
传统上认为,强布朗斯台德酸溶剂中的 Sb(V) 通过超酸质子分解与轻质烷烃反应,产生碳正离子中间体 H 2, 和碳齐聚。与这一一般假设相反,我们的密度泛函理论 (DFT) 计算揭示了硫酸中甲烷和 Sb(V) 之间 C-H 活化的可接近障碍,这可能会超过超酸质子分解。这促使我们通过实验检查硫酸与发烟硫酸中的这种反应,由于假定的超酸反应性而从未报道过。甲烷在 180 °C 下反应 3 小时导致非常高的硫酸氢甲酯产率,而没有明显的过氧化。我们的 DFT 计算表明,产生硫酸氢甲酯的 C-H 活化和 Sb-Me 键官能化机制胜过甲烷质子分解和许多其他可能的反应机制,例如电子转移、质子耦合电子转移和氢化物提取。我们的 DFT 计算还解释了实验性氢-氘交换研究以及不存在甲烷碳官能化/低聚产物。总体而言,这项工作表明,在非常强的布朗斯台德酸溶剂中,Sb(V) 可以诱导类似于过渡金属的内层反应机制,并超过超强酸的反应性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号