当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homocoupling of a Grignard Reagent toward the Synthesis of 2,2′-Bis(trifluoromethyl)-4,4′-diaminobiphenyl
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-10-19 , DOI: 10.1021/acs.oprd.1c00227 Jiro Nakatani 1 , Tatsuhiro Nozoe 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-10-19 , DOI: 10.1021/acs.oprd.1c00227 Jiro Nakatani 1 , Tatsuhiro Nozoe 1
Affiliation
|
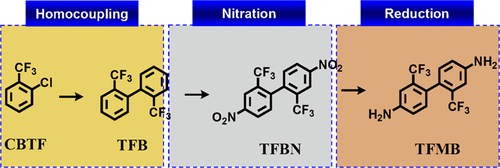
A new process for the synthesis of 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl (TFMB) comprising three steps has been industrially developed. The first step is the homocoupling of the Grignard reagent derived from 1-chloro-2-(trifluoromethyl)benzene with iron chloride(III) in the presence of an oxidizing agent to afford 2,2′-bis(trifluoromethyl)biphenyl. The second step is the nitration of this biphenyl to 2,2′-bis(trifluoromethyl)-4,4′-dinitrobiphenyl, and the third step is the reduction of this dinitrobiphenyl using hydrogen over Pd/C to afford TFMB. This process has been demonstrated on an industrial scale.
中文翻译:

用于合成 2,2'-双(三氟甲基)-4,4'-二氨基联苯的格氏试剂的同源偶联
工业上开发了一种合成 2,2'-双(三氟甲基)-4,4'-二氨基联苯 (TFMB) 的新工艺,包括三个步骤。第一步是在氧化剂存在下将源自 1-氯-2-(三氟甲基)苯的格氏试剂与氯化铁(III)均偶联,得到 2,2'-双(三氟甲基)联苯。第二步是将该联苯硝化为 2,2'-双(三氟甲基)-4,4'-二硝基联苯,第三步是使用氢气在 Pd/C 上还原该二硝基联苯以提供 TFMB。该方法已在工业规模上得到证明。
更新日期:2021-11-19
中文翻译:

用于合成 2,2'-双(三氟甲基)-4,4'-二氨基联苯的格氏试剂的同源偶联
工业上开发了一种合成 2,2'-双(三氟甲基)-4,4'-二氨基联苯 (TFMB) 的新工艺,包括三个步骤。第一步是在氧化剂存在下将源自 1-氯-2-(三氟甲基)苯的格氏试剂与氯化铁(III)均偶联,得到 2,2'-双(三氟甲基)联苯。第二步是将该联苯硝化为 2,2'-双(三氟甲基)-4,4'-二硝基联苯,第三步是使用氢气在 Pd/C 上还原该二硝基联苯以提供 TFMB。该方法已在工业规模上得到证明。































 京公网安备 11010802027423号
京公网安备 11010802027423号