当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal-Structure-Dependent Photocatalytic Redox Activity and Reaction Pathways over Ga2O3 Polymorphs
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-10-19 , DOI: 10.1021/acsami.1c14920
Peng Chen 1, 2 , Kanglu Li 2, 3 , Ben Lei 2 , Lvcun Chen 2 , Wen Cui 1 , Yanjuan Sun 2 , Wendong Zhang 4 , Ying Zhou 1 , Fan Dong 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-10-19 , DOI: 10.1021/acsami.1c14920
Peng Chen 1, 2 , Kanglu Li 2, 3 , Ben Lei 2 , Lvcun Chen 2 , Wen Cui 1 , Yanjuan Sun 2 , Wendong Zhang 4 , Ying Zhou 1 , Fan Dong 1, 2
Affiliation
|
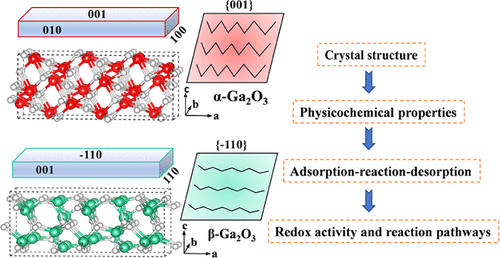
Differentiated crystal structures generally affect the surface physicochemical properties of catalysts, causing variety in catalytic activity between polymorphs. However, the underlying mechanism has not been completely revealed, especially the influence of surface physicochemical properties on photocatalytic redox activity and the reaction mechanism. In this work, we reveal the mechanism of surface redox properties on different crystal forms of gallium oxide from a molecular level. α-Ga2O3 and β-Ga2O3 exhibit a slight difference in catalytic oxidation of organic pollutants due to comprehensive influencing factors, including their valence band position, reactive oxygen species, and pore structure properties related to the adsorption–reaction–desorption process. But the catalytic reduction ability of CO2 is obviously different due to the large differences of interaction between the surface of crystal structures and CO2 molecules, which are critical to determine the catalytic performance and reaction pathways. The enhanced adsorption and activation of CO2 on the α-Ga2O3 surface could promote the reduction reaction efficiency. Moreover, the large energy barrier of CH2* formation on β-Ga2O3 makes the formation of methane (CH4) relatively difficult compared to that on α-Ga2O3. The yield rate of CH4 (1.8 μmol·g–1·h–1) on α-Ga2O3 is three times better than that on β-Ga2O3 (CH4: 0.6 μmol·g–1·h–1). The current findings can offer novel insights into the understanding of crystal-structure-dependent photocatalytic performances and the design of new catalysts applied in energy conversion and environmental purification by crystal structure-tuning.
中文翻译:

晶体结构相关的光催化氧化还原活性和 Ga2O3 多晶型物的反应途径
不同的晶体结构通常会影响催化剂的表面物理化学性质,导致多晶型物之间催化活性的差异。然而,其潜在机制尚未完全揭示,特别是表面理化性质对光催化氧化还原活性和反应机理的影响。在这项工作中,我们从分子水平揭示了不同晶型氧化镓的表面氧化还原特性的机制。α-Ga 2 O 3和β-Ga 2 O 3由于综合影响因素,包括价带位置、活性氧物种以及与吸附-反应-解吸过程相关的孔结构特性,有机污染物的催化氧化表现出轻微差异。但由于晶体结构表面与CO 2分子之间的相互作用差异较大,CO 2的催化还原能力明显不同,这对决定催化性能和反应途径至关重要。CO 2在α-Ga 2 O 3表面的增强吸附和活化可以提高还原反应效率。此外,CH 2的大能垒*与α-Ga 2 O 3相比,β-Ga 2 O 3上的形成使得甲烷(CH 4)的形成相对困难。CH 4 (1.8 μmol·g –1 ·h –1 )在α-Ga 2 O 3上的产率是β-Ga 2 O 3 (CH 4 : 0.6 μmol·g –1 ·h )上的三倍–1)。目前的发现可以为理解依赖于晶体结构的光催化性能的理解以及通过晶体结构调整应用于能量转换和环境净化的新催化剂的设计提供新的见解。
更新日期:2021-11-03
中文翻译:

晶体结构相关的光催化氧化还原活性和 Ga2O3 多晶型物的反应途径
不同的晶体结构通常会影响催化剂的表面物理化学性质,导致多晶型物之间催化活性的差异。然而,其潜在机制尚未完全揭示,特别是表面理化性质对光催化氧化还原活性和反应机理的影响。在这项工作中,我们从分子水平揭示了不同晶型氧化镓的表面氧化还原特性的机制。α-Ga 2 O 3和β-Ga 2 O 3由于综合影响因素,包括价带位置、活性氧物种以及与吸附-反应-解吸过程相关的孔结构特性,有机污染物的催化氧化表现出轻微差异。但由于晶体结构表面与CO 2分子之间的相互作用差异较大,CO 2的催化还原能力明显不同,这对决定催化性能和反应途径至关重要。CO 2在α-Ga 2 O 3表面的增强吸附和活化可以提高还原反应效率。此外,CH 2的大能垒*与α-Ga 2 O 3相比,β-Ga 2 O 3上的形成使得甲烷(CH 4)的形成相对困难。CH 4 (1.8 μmol·g –1 ·h –1 )在α-Ga 2 O 3上的产率是β-Ga 2 O 3 (CH 4 : 0.6 μmol·g –1 ·h )上的三倍–1)。目前的发现可以为理解依赖于晶体结构的光催化性能的理解以及通过晶体结构调整应用于能量转换和环境净化的新催化剂的设计提供新的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号