European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-10-19 , DOI: 10.1016/j.ejmech.2021.113933 Mengze Zhou 1 , Weiwei Wang 2 , Zhongkui Wang 3 , Yilin Wang 2 , Yifan Zhu 2 , Zhiqian Lin 2 , Sheng Tian 2 , Yuan Huang 2 , Qinghua Hu 4 , Huanqiu Li 2
|
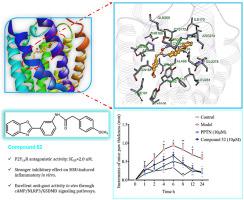
The P2Y14 nucleotide receptor, a subtype of P2Y receptors, is implicated in many human inflammatory diseases. Based on the identification of favorable residues of two screening hits in the almost symmetrical P2Y14 binding domain, we describe the structural optimization of previously identified virtual screening hits 6 and 7 that result in the development of P2Y14R antagonists with a novel 2-phenyl-benzoxazole acetamide chemical scaffold. Notably, compound 52 showed potent P2Y14R antagonistic activity (IC50 = 2 nM), and a stronger inhibitory effect on MSU-induced inflammatory in vitro, better than a previously described P2Y14R antagonist PPTN. In vivo evaluation demonstrated that compound 52 also had satisfactory inhibitory activity on the inflammatory response of gout flares in mice. Moreover, P2Y14R antagonist 52 decreased paw swelling and inflammatory cell infiltration through cAMP/NLRP3/GSDMD signaling pathways in MSU-induced acute gouty arthritis mice. The discussions on the binding mechanism that employ MM/GBSA free energy calculations/decompositions also provide some useful clues for further structural designing of compound 52. Taken together, 2-phenyl-benzoxazole acetamide derivative 52 with potent P2Y14R antagonistic activity and in vivo potency could be a promising strategy for gout therapy and deserves further optimization.
中文翻译:

2-苯基-苯并恶唑乙酰胺衍生物作为具有抗痛风潜力的有前景的 P2Y14R 拮抗剂的发现和计算研究
P2Y 14核苷酸受体是 P2Y 受体的一种亚型,与许多人类炎症性疾病有关。基于对几乎对称的 P2Y 14结合域中两个筛选命中的有利残基的鉴定,我们描述了先前确定的虚拟筛选命中6和7的结构优化,导致开发具有新型 2-苯基的 P2Y 14 R 拮抗剂-苯并恶唑乙酰胺化学支架。值得注意的是,化合物52显示出有效的 P2Y 14 R 拮抗活性 (IC 50 = 2 nM),并且在体外 对 MSU 诱导的炎症具有更强的抑制作用,优于先前描述的 P2Y 14 R 拮抗剂 PPTN。体内评估表明,化合物52对小鼠痛风发作的炎症反应也具有令人满意的抑制活性。此外,P2Y 14 R 拮抗剂52通过 cAMP/NLRP3/GSDMD 信号通路减少 MSU 诱导的急性痛风性关节炎小鼠的爪肿胀和炎症细胞浸润。对采用 MM/GBSA 自由能计算/分解的结合机制的讨论也为化合物52的进一步结构设计提供了一些有用的线索。总之,2-苯基-苯并恶唑乙酰胺衍生物52与强效 P2Y 14R 拮抗活性和体内效力可能是痛风治疗的一种有前途的策略,值得进一步优化。







































 京公网安备 11010802027423号
京公网安备 11010802027423号