当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of 1H-Triazole-1-[N,N′-Bis(tert-butoxycarbonyl)]carboxamidine in Solution-Phase and On-Resin Guanidinylation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-10-18 , DOI: 10.1021/acs.joc.1c00994 Anita Wester 1 , Fredrik Björkling 1 , Henrik Franzyk 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-10-18 , DOI: 10.1021/acs.joc.1c00994 Anita Wester 1 , Fredrik Björkling 1 , Henrik Franzyk 1
Affiliation
|
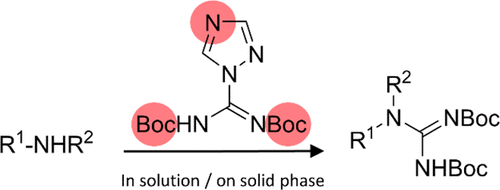
Several guanidines and guanidinylated peptides have substantial potential as therapeutics, but efficient guanidinylation reagents are vital for easy access to these compounds. Presently, pyrazole-1-carboxamidine type reagents are commonly used in the transformations of amines into corresponding guanidines. Here, we report a comparative study of the utility of 1H-triazole-1-[N,N′-bis(tert-butoxycarbonyl)]carboxamidine, which was synthesized in two steps and readily upscaled to gram amounts. It exhibited excellent performance in solution-phase reactions, rapidly converting a set of representative aliphatic primary and unhindered secondary amines as well as aniline into the corresponding bis(tert-butoxycarbonyl)-protected guanidines. To enable a direct assessment of the reactivity of guanidinylation reagents, conversions were performed in deuterated solvents (d7-DMF or d8-THF), allowing for continuous analysis of the reaction mixtures by 1H and 13C NMR. Likewise, 1H-triazole-1-[N,N′-bis(tert-butoxycarbonyl)]carboxamidine proved to be a versatile reagent in solid-phase conversions, for example, a resin-bound test peptide (KFFKFFK) was fully guanidinylated in only 2 h by using 2 equivalents of the reagent per free amino group. Also, 1H-triazole-1-[N,N′-bis(tert-butoxycarbonyl)]carboxamidine proved capable of completely guanidinylating more sterically hindered N-terminal residues (e.g., N-methyl amino acids or a peptoid) in resin-bound peptides. Its superior reactivity and stability demonstrated under heating conditions make 1H-triazole-1-[N,N′-bis(tert-butoxycarbonyl)]carboxamidine a valuable guanidinylation reagent both in solution- and solid-phase synthesis.
中文翻译:

1H-三唑-1-[N,N'-双(叔丁氧基羰基)]甲脒在溶液相和树脂上胍基化中的评价
几种胍和胍基化肽具有巨大的治疗潜力,但有效的胍基化试剂对于容易获得这些化合物至关重要。目前,吡唑-1-甲脒类试剂常用于将胺类转化为相应的胍类。在这里,我们报告了 1 H-三唑-1-[ N , N '-双(叔丁氧基羰基)]甲脒的效用的比较研究,该化合物分两步合成并易于放大至克量。它在液相反应中表现出优异的性能,可将一组具有代表性的脂肪族伯胺和未受阻仲胺以及苯胺迅速转化为相应的双(叔胺)-丁氧羰基)-保护的胍。为了能够直接评估胍基化试剂的反应性,在氘代溶剂(d 7 -DMF 或d 8 -THF)中进行转化,从而允许通过1 H 和13 C NMR对反应混合物进行连续分析。同样,1 H -triazole-1-[ N , N '-bis( tert -butoxycarbonyl)] 甲脒被证明是固相转化中的通用试剂,例如,树脂结合的测试肽 (KFFKFFK) 被完全胍基化每个游离氨基使用 2 当量的试剂仅需 2 小时。此外, 1 H -triazole-1-[ N, N'-双(叔丁氧基羰基)]甲脒证明能够完全胍基化树脂结合肽中空间位阻更大的N-末端残基(例如,N-甲基氨基酸或类肽)。其在加热条件下表现出的优异反应性和稳定性使 1 H-三唑-1-[ N , N '-双(叔丁氧基羰基)]甲脒在溶液和固相合成中都是一种有价值的胍基化试剂。
更新日期:2021-11-05
中文翻译:

1H-三唑-1-[N,N'-双(叔丁氧基羰基)]甲脒在溶液相和树脂上胍基化中的评价
几种胍和胍基化肽具有巨大的治疗潜力,但有效的胍基化试剂对于容易获得这些化合物至关重要。目前,吡唑-1-甲脒类试剂常用于将胺类转化为相应的胍类。在这里,我们报告了 1 H-三唑-1-[ N , N '-双(叔丁氧基羰基)]甲脒的效用的比较研究,该化合物分两步合成并易于放大至克量。它在液相反应中表现出优异的性能,可将一组具有代表性的脂肪族伯胺和未受阻仲胺以及苯胺迅速转化为相应的双(叔胺)-丁氧羰基)-保护的胍。为了能够直接评估胍基化试剂的反应性,在氘代溶剂(d 7 -DMF 或d 8 -THF)中进行转化,从而允许通过1 H 和13 C NMR对反应混合物进行连续分析。同样,1 H -triazole-1-[ N , N '-bis( tert -butoxycarbonyl)] 甲脒被证明是固相转化中的通用试剂,例如,树脂结合的测试肽 (KFFKFFK) 被完全胍基化每个游离氨基使用 2 当量的试剂仅需 2 小时。此外, 1 H -triazole-1-[ N, N'-双(叔丁氧基羰基)]甲脒证明能够完全胍基化树脂结合肽中空间位阻更大的N-末端残基(例如,N-甲基氨基酸或类肽)。其在加热条件下表现出的优异反应性和稳定性使 1 H-三唑-1-[ N , N '-双(叔丁氧基羰基)]甲脒在溶液和固相合成中都是一种有价值的胍基化试剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号