当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Delivery and Site-Specific Activation of β-Cyclodextrin-Conjugated Photosensitizers for Photodynamic Therapy through a Supramolecular Bio-orthogonal Approach
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-10-18 , DOI: 10.1021/acs.jmedchem.1c01505 Evelyn Y Xue 1 , Wen-Jing Shi 1 , Wing-Ping Fong 2 , Dennis K P Ng 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-10-18 , DOI: 10.1021/acs.jmedchem.1c01505 Evelyn Y Xue 1 , Wen-Jing Shi 1 , Wing-Ping Fong 2 , Dennis K P Ng 1
Affiliation
|
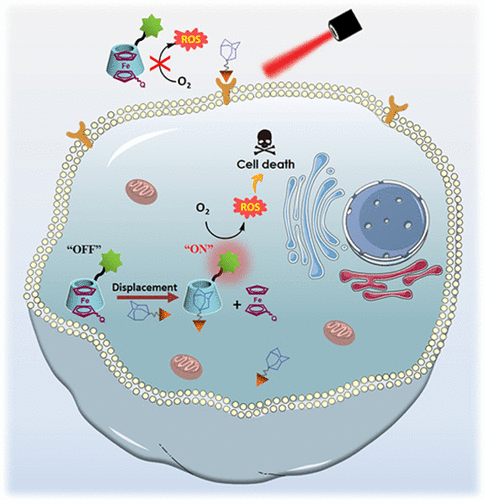
Targeted delivery of photosensitizers using hydrophilic and tumor-directing carriers and site-specific activation of their photocytotoxicity are two common strategies to enhance the specificity of anticancer photodynamic therapy. We report herein a novel supramolecular bio-orthogonal approach to integrate these two functions. A β-cyclodextrin-substituted aza-boron-dipyrromethene-based photosensitizer was first complexed with a ferrocene-substituted black-hole quencher to inhibit its photosensitizing ability. Upon encountering the adamantane moieties that had been delivered to target cancer cells through specific binding of the conjugated peptide to the overexpressed epidermal growth factor receptor, the ferrocene-based guest species were displaced due to the stronger binding interactions between β-cyclodextrin and adamantane, thereby restoring the photodynamic activity of the photosensitizer. Hence, this two-step process enabled targeted delivery and site-specific activation of the photosensitizer, as demonstrated through a series of experiments in aqueous media, in a range of cancer cell lines and in tumor-bearing nude mice.
中文翻译:

通过超分子生物正交方法靶向递送和位点特异性激活用于光动力治疗的 β-环糊精偶联光敏剂
使用亲水性和肿瘤导向载体靶向递送光敏剂以及对其光细胞毒性的位点特异性激活是增强抗癌光动力疗法特异性的两种常见策略。我们在此报告了一种新的超分子生物正交方法来整合这两个功能。β-环糊精取代的氮杂-硼-二吡咯甲烯基光敏剂首先与二茂铁取代的黑洞猝灭剂复合以抑制其光敏能力。在遇到通过结合肽与过度表达的表皮生长因子受体的特异性结合而被递送至靶癌细胞的金刚烷部分时,基于二茂铁的客体物种由于β-环糊精和金刚烷之间更强的结合相互作用而被取代,从而恢复光敏剂的光动力活性。因此,正如在水性介质、一系列癌细胞系和荷瘤裸鼠中的一系列实验所证明的那样,这种两步过程能够实现光敏剂的靶向递送和位点特异性激活。
更新日期:2021-10-28
中文翻译:

通过超分子生物正交方法靶向递送和位点特异性激活用于光动力治疗的 β-环糊精偶联光敏剂
使用亲水性和肿瘤导向载体靶向递送光敏剂以及对其光细胞毒性的位点特异性激活是增强抗癌光动力疗法特异性的两种常见策略。我们在此报告了一种新的超分子生物正交方法来整合这两个功能。β-环糊精取代的氮杂-硼-二吡咯甲烯基光敏剂首先与二茂铁取代的黑洞猝灭剂复合以抑制其光敏能力。在遇到通过结合肽与过度表达的表皮生长因子受体的特异性结合而被递送至靶癌细胞的金刚烷部分时,基于二茂铁的客体物种由于β-环糊精和金刚烷之间更强的结合相互作用而被取代,从而恢复光敏剂的光动力活性。因此,正如在水性介质、一系列癌细胞系和荷瘤裸鼠中的一系列实验所证明的那样,这种两步过程能够实现光敏剂的靶向递送和位点特异性激活。































 京公网安备 11010802027423号
京公网安备 11010802027423号