Hydrometallurgy ( IF 4.8 ) Pub Date : 2021-10-16 , DOI: 10.1016/j.hydromet.2021.105768 Jinqing Chen 1 , Hepeng Zhang 1, 2, 3 , Zhiyuan Zeng 2, 3 , Yun Gao 2, 3 , Chenhao Liu 1, 2, 3 , Xiaoqi Sun 2, 3, 4
|
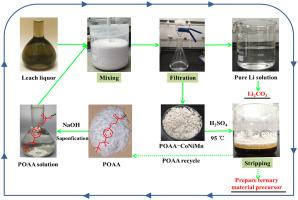
Unlike common solvent extraction and chemical precipitation, a novel extraction-precipitation process for the separation of Li and transition metals using p-tertylphenoxyacetic acid (POAA) is developed. Extraction-precipitation method refers to a method in which the extraction-precipitant can quantitatively extract the metal ions to form solid extracted complex without the requirement of organic solvent or carrier, and the extraction-precipitant can be stripped and recycled. In this study, the extraction-precipitation mechanism and thermodynamic parameters between POAA and manganese (Mn), cobalt (Co), nickel (Ni) were analyzed. Subsequently, the equilibrium time and pH value of the separation process were optimized. Finally, the leach solution of LIB having Li, Ni, Co, Mn with concentration of 3.52 g/L, 7.93 g/L, 5.72 g/L, and 14.16 g/L, respectively, was studied for the separation at pH 4.5. The results indicate that efficient separation of Li and transition metals was possible by one-step precipitation using 0.2 mol/L POAA solution (degree of saponification = 100%) when the ratio of nPOAA/nMn+Co+Ni was 2. The separation factor for the transition metals over lithium (βCoNiMn/βLi) reached as high as 133,014. After the transition metal-loaded POAA was stripped with 2 mol/L sulfuric acid, the stripped solution could be used for the production of ternary material precursor, and the regenerated POAA could be recycled for the extraction-precipitation process. The lithium in the solution was recovered in the form of lithium carbonate (Li2CO3) with a purity level of 97.7%. This high efficiency and sustainable process based on extraction-precipitation has shown potential application for spent LIB recovery.
中文翻译:

对叔丁基苯氧基乙酸萃取-沉淀法从废锂离子电池浸出液中分离锂和过渡金属
与常见的溶剂萃取和化学沉淀不同,本文开发了一种使用对叔苯氧乙酸 (POAA) 分离锂和过渡金属的新型萃取-沉淀工艺。萃取-沉淀法是指无需有机溶剂或载体,萃取-沉淀剂可定量萃取金属离子,形成固体萃取络合物,萃取-沉淀剂可汽提回收利用的方法。本研究分析了POAA与锰(Mn)、钴(Co)、镍(Ni)的萃取-沉淀机理和热力学参数。随后,优化了分离过程的平衡时间和pH值。最后,具有浓度为3.52g/L、7.93g/L、5.72g/L和14.16g/L的Li、Ni、Co、Mn的LIB浸出液,分别研究了在 pH 4.5 下的分离。结果表明,当 nPOAA /n Mn+Co+Ni为2。过渡金属与锂的分离系数(β CoNiMn / β Li)高达133,014。负载过渡金属的POAA用2mol/L硫酸汽提后,汽提后的溶液可用于生产三元材料前驱体,再生后的POAA可循环用于萃取-沉淀过程。溶液中的锂以碳酸锂(Li 2 CO 3)的形式回收,纯度为97.7%。这种基于提取-沉淀的高效和可持续过程已显示出废LIB回收的潜在应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号