Journal of Rare Earths ( IF 5.2 ) Pub Date : 2021-10-16 , DOI: 10.1016/j.jre.2021.10.001
Rabia Kırkgeçit 1 , Handan Özlü Torun 2 , Fatma Kılıç Dokan 3 , Esra Öztürk 4
|
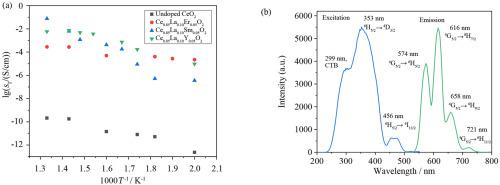
In this research, un-doped CeO2 and Ce0.85La0.10M0.05O2 (M: Sm, Er, Y) compounds were synthesized by hydrothermal method and the multi-functional properties are reported. Oxygen defects were created with the additives of rare earth ions. The electrical and luminescence behaviors of the synthesized compounds were investigated in accord with the types of additives. The synthesized products were characterized by X-ray diffraction (XRD) analysis, Brunauer–Emmett–Teller (BET) measurement, UV–vis diffuse reflectance spectroscopy (DRS), scanning electron microscopy (SEM), photoluminescence (PL) spectroscopy and electrochemical impedance spectroscopic (EIS). All synthesized compounds are found to be nano-structured and have cubic phase. The total conductivity of all samples was calculated. Hence, the total conductivity of un-doped CeO2, Ce0.85La0.10Y0.05O2, Ce0.85La0.10Er0.05O2 and Ce0.85La0.10Sm0.05O2 is found to be 2.07 × 10−10, 5.70 × 10−4, 1.0 × 10−3 and 0.0747 S/cm, respectively. Also, bandgap energy (Eg) of these samples calculated from UV visible absorption spectra is discussed, and the optical results show variation between 3.2 and 2.15 eV. Additionally, the luminescence properties of the compounds were investigated and different emissions occur depending on the additive type. Accordingly, photoluminescent emission spectra of Ce0.85La0.10Y0.05O2, Ce0.85La0.10Er0.05O2 and Ce0.85La0.10Sm0.05O2 phosphors indicate that these phosphors have red, green and orange-red colors, respectively.
中文翻译:

稀土元素(Sm、Y、La、Er)共掺杂CeO2的光学和导电性能
在本研究中,未掺杂的 CeO 2和 Ce 0.85 La 0.10 M 0.05 O 2(M: Sm, Er, Y) 化合物采用水热法合成,并报道了其多功能性质。氧缺陷是由稀土离子的添加剂产生的。根据添加剂的类型研究了合成化合物的电学和发光行为。通过 X 射线衍射 (XRD) 分析、Brunauer-Emmett-Teller (BET) 测量、紫外-可见漫反射光谱 (DRS)、扫描电子显微镜 (SEM)、光致发光 (PL) 光谱和电化学阻抗对合成产物进行表征光谱(EIS)。发现所有合成的化合物都是纳米结构的并具有立方相。计算所有样品的总电导率。因此,未掺杂CeO 2的总电导率,Ce 0.85 La0.10 Y 0.05 O 2、Ce 0.85 La 0.10 Er 0.05 O 2和Ce 0.85 La 0.10 Sm 0.05 O 2为2.07×10 -10、5.70×10 -4、1.0×10 -3和0.0747 S / cm,分别。此外,带隙能量(E g) 讨论了从紫外可见吸收光谱计算的这些样品,光学结果显示在 3.2 和 2.15 eV 之间的变化。此外,还研究了化合物的发光特性,并根据添加剂类型发生不同的发射。因此,Ce 0.85 La 0.10 Y 0.05 O 2、Ce 0.85 La 0.10 Er 0.05 O 2和Ce 0.85 La 0.10 Sm 0.05 O 2荧光粉的光致发光发射光谱表明这些荧光粉分别具有红色、绿色和橙红色。



































 京公网安备 11010802027423号
京公网安备 11010802027423号