当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Syntheses of Vicinal Dichloride Monoterpenes Enabled by Aza-Belluš–Claisen Rearrangement
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.orglett.1c03187 Jiangqun Cheng 1 , Yuan-He Li 2 , Jun Huang 1 , Zhen Yang 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.orglett.1c03187 Jiangqun Cheng 1 , Yuan-He Li 2 , Jun Huang 1 , Zhen Yang 1, 2
Affiliation
|
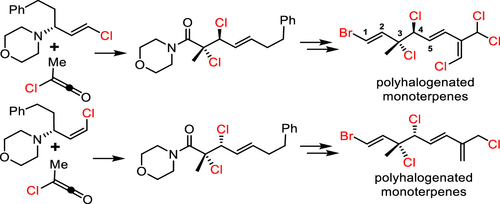
Diastereoselective syntheses of syn- and anti-vicinal dihalides were achieved via an aza-Belluš–Claisen rearrangement, which involved the reaction of an α-chloro carboxylic acid chloride with halogen-substituted trans-allyl morpholines in the presence of Lewis acids. The developed method was used for the total synthesis of a group of monoterpene natural products bearing vicinal dichloride subunits.
中文翻译:

由 Aza-Belluš–Claisen 重排实现的邻二氯单萜的全合成
顺和反邻位二卤化物的非对映选择性合成是通过氮杂-贝卢什-克莱森重排实现的,该重排涉及在路易斯酸存在下,α-氯代羧酸氯化物与卤素取代的反式烯丙基吗啉反应。所开发的方法用于全合成一组带有连二氯亚基的单萜天然产物。
更新日期:2021-11-05
中文翻译:

由 Aza-Belluš–Claisen 重排实现的邻二氯单萜的全合成
顺和反邻位二卤化物的非对映选择性合成是通过氮杂-贝卢什-克莱森重排实现的,该重排涉及在路易斯酸存在下,α-氯代羧酸氯化物与卤素取代的反式烯丙基吗啉反应。所开发的方法用于全合成一组带有连二氯亚基的单萜天然产物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号