当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
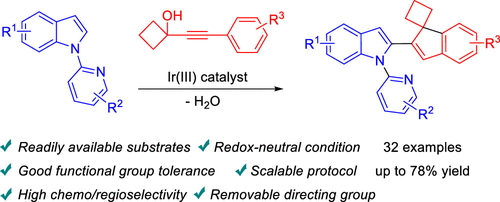
Synthesis of Indolyl-Tethered Spiro[cyclobutane-1,1′-indenes] through Cascade Reactions of 1-(Pyridin-2-yl)-1H-indoles with Alkynyl Cyclobutanols
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.orglett.1c03200 Yuanshuang Xu 1 , Caiyun Yu 1 , Xinying Zhang 1 , Xuesen Fan 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.orglett.1c03200 Yuanshuang Xu 1 , Caiyun Yu 1 , Xinying Zhang 1 , Xuesen Fan 1
Affiliation
|
Presented herein is an efficient and unprecedented synthesis of indolyl-tethered spiro[cyclobutane-1,1′-indenes] through the cascade reaction of 1-(pyridin-2-yl)-1H-indoles with alkynyl cyclobutanols. Mechanistic experiments implicate a sequential process in which 1-(pyridin-2-yl)-1H-indole first undergoes an alkenylation with alkynyl cyclobutanol followed by an intramolecular Friedel–Crafts reaction to give the title products. The utility of this novel protocol was reflected by the ample substrate scope, high chemo- and regioselectivity, removable directing group, and scalable preparation. In addition, the product thus obtained can be further derivatized quite efficiently.
中文翻译:

通过 1-(吡啶-2-基)-1H-吲哚与炔基环丁醇的级联反应合成吲哚基连接的螺[环丁烷-1,1'-茚]
本文介绍了通过 1-(吡啶-2-基)-1 H-吲哚与炔基环丁醇的级联反应,高效且前所未有地合成吲哚基栓系的螺[环丁烷-1,1'-茚] 。机理实验涉及一个连续过程,其中 1-(pyridin-2-yl)-1 H-吲哚首先与炔基环丁醇进行烯基化,然后进行分子内 Friedel-Crafts 反应,得到标题产物。这种新协议的效用体现在充足的底物范围、高化学和区域选择性、可移除的导向基团和可扩展的制备。此外,由此获得的产品可以非常有效地进一步衍生化。
更新日期:2021-11-05
中文翻译:

通过 1-(吡啶-2-基)-1H-吲哚与炔基环丁醇的级联反应合成吲哚基连接的螺[环丁烷-1,1'-茚]
本文介绍了通过 1-(吡啶-2-基)-1 H-吲哚与炔基环丁醇的级联反应,高效且前所未有地合成吲哚基栓系的螺[环丁烷-1,1'-茚] 。机理实验涉及一个连续过程,其中 1-(pyridin-2-yl)-1 H-吲哚首先与炔基环丁醇进行烯基化,然后进行分子内 Friedel-Crafts 反应,得到标题产物。这种新协议的效用体现在充足的底物范围、高化学和区域选择性、可移除的导向基团和可扩展的制备。此外,由此获得的产品可以非常有效地进一步衍生化。







































 京公网安备 11010802027423号
京公网安备 11010802027423号