当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Epoxyqueuosine Reductase QueH in the Biosynthetic Pathway to tRNA Queuosine Is a Unique Metalloenzyme
Biochemistry ( IF 2.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.biochem.1c00164 Qiang Li 1 , Rémi Zallot 2 , Brian S MacTavish 1 , Alvaro Montoya 1 , Daniel J Payan 2 , You Hu 1 , John A Gerlt 2, 3 , Alexander Angerhofer 1 , Valérie de Crécy-Lagard 4, 5 , Steven D Bruner 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acs.biochem.1c00164 Qiang Li 1 , Rémi Zallot 2 , Brian S MacTavish 1 , Alvaro Montoya 1 , Daniel J Payan 2 , You Hu 1 , John A Gerlt 2, 3 , Alexander Angerhofer 1 , Valérie de Crécy-Lagard 4, 5 , Steven D Bruner 1
Affiliation
|
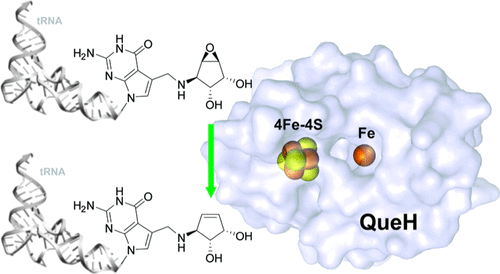
Queuosine is a structurally unique and functionally important tRNA modification, widely distributed in eukaryotes and bacteria. The final step of queuosine biosynthesis is the reduction/deoxygenation of epoxyqueuosine to form the cyclopentene motif of the nucleobase. The chemistry is performed by the structurally and functionally characterized cobalamin-dependent QueG. However, the queG gene is absent from several bacteria that otherwise retain queuosine biosynthesis machinery. Members of the IPR003828 family (previously known as DUF208) have been recently identified as nonorthologous replacements of QueG, and this family was renamed QueH. Here, we present the structural characterization of QueH from Thermotoga maritima. The structure reveals an unusual active site architecture with a [4Fe-4S] metallocluster along with an adjacent coordinated iron metal. The juxtaposition of the cofactor and coordinated metal ion predicts a unique mechanism for a two-electron reduction/deoxygenation of epoxyqueuosine. To support the structural characterization, in vitro biochemical and genomic analyses are presented. Overall, this work reveals new diversity in the chemistry of iron/sulfur-dependent enzymes and novel insight into the last step of this widely conserved tRNA modification.
中文翻译:

tRNA 的生物合成途径中的环氧奎因还原酶 QueH 是一种独特的金属酶
Queuosine 是一种结构独特且功能重要的 tRNA 修饰,广泛分布于真核生物和细菌中。奎奥辛生物合成的最后一步是环氧奎奥辛还原/脱氧以形成核碱基的环戊烯基序。化学由结构和功能表征的钴胺素依赖性 QueG 进行。然而,queG基因在一些细菌中不存在,否则这些细菌会保留奎奥苷生物合成机制。IPR003828 家族(以前称为 DUF208)的成员最近被确定为 QueG 的非直系同源替代品,该家族被重新命名为 QueH。在这里,我们介绍了来自Thermotoga maritima的 QueH 的结构表征. 该结构揭示了一种不寻常的活性位点结构,具有 [4Fe-4S] 金属簇和相邻的配位铁金属。辅因子和配位金属离子的并置预测了环氧奎奥辛双电子还原/脱氧的独特机制。为了支持结构表征,提出了体外生化和基因组分析。总体而言,这项工作揭示了铁/硫依赖性酶化学的新多样性,以及对这种广泛保守的 tRNA 修饰的最后一步的新见解。
更新日期:2021-10-26
中文翻译:

tRNA 的生物合成途径中的环氧奎因还原酶 QueH 是一种独特的金属酶
Queuosine 是一种结构独特且功能重要的 tRNA 修饰,广泛分布于真核生物和细菌中。奎奥辛生物合成的最后一步是环氧奎奥辛还原/脱氧以形成核碱基的环戊烯基序。化学由结构和功能表征的钴胺素依赖性 QueG 进行。然而,queG基因在一些细菌中不存在,否则这些细菌会保留奎奥苷生物合成机制。IPR003828 家族(以前称为 DUF208)的成员最近被确定为 QueG 的非直系同源替代品,该家族被重新命名为 QueH。在这里,我们介绍了来自Thermotoga maritima的 QueH 的结构表征. 该结构揭示了一种不寻常的活性位点结构,具有 [4Fe-4S] 金属簇和相邻的配位铁金属。辅因子和配位金属离子的并置预测了环氧奎奥辛双电子还原/脱氧的独特机制。为了支持结构表征,提出了体外生化和基因组分析。总体而言,这项工作揭示了铁/硫依赖性酶化学的新多样性,以及对这种广泛保守的 tRNA 修饰的最后一步的新见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号