Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-10-14 , DOI: 10.1016/j.cej.2021.132917 Xiaohai Zheng 1 , Yanli Li 2 , Weilong You 1 , Ganchang Lei 1 , Yanning Cao 1, 3 , Yongfan Zhang 2 , Lilong Jiang 1, 3
|
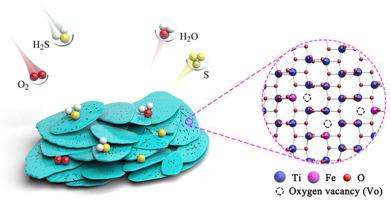
Introduction of surface oxygen vacancy (Vo) has been proved to be a powerful method to promote the performance of H2S selective oxidation by improving H2S adsorption and O2 activation. Nevertheless, maximizing the oxygen vacancy concentration remains a challenge due to limited exposed surface. Herein, we report a Fe-doped TiO2-x ultrathin nanosheet with abundant oxygen vacancies for H2S selective oxidation via a facile citric acid assisted hydrothermal process. One of the cheapest and most abundant metals, iron, is a desirable dopant for further promoting the H2S oxidation activity of TiO2. As a result, the Fe-doped TiO2-x nanosheets endowed with abundant oxygen vacancies exhibited nearly 100% sulfur selectivity and H2S conversion at 210 °C and is superior to most reported Ti-based materials. Furthermore, through in situ DRIFTS, in situ Raman and EPR spectra of H2S oxidation, the reaction pathway in selective oxidation of H2S is revealed. The density functional theory (DFT) calculation was conducted to get a deeper insight into the effect of Fe-doping on the electronic structure and oxygen vacancy of defected TiO2.
中文翻译:

构建具有丰富氧空位的 Fe 掺杂 TiO2-x 超薄纳米片以高效氧化 H2S
表面氧空位(VO)的引入已被证明是促进H的性能的有效方法2通过改善小时秒选择性氧化2小号吸附和O 2活化。然而,由于有限的暴露表面,最大化氧空位浓度仍然是一个挑战。在此,我们报告了一种 Fe 掺杂的 TiO 2-x超薄纳米片,其具有丰富的氧空位,可通过简单的柠檬酸辅助水热过程进行 H 2 S 选择性氧化。铁是最便宜和最丰富的金属之一,是进一步促进TiO 2的 H 2 S 氧化活性的理想掺杂剂。因此,Fe 掺杂的 TiO 2-x具有丰富氧空位的纳米片在 210°C 时表现出接近 100% 的硫选择性和 H 2 S 转化率,并且优于大多数报道的钛基材料。此外,通过H 2 S氧化的原位漂移、原位拉曼和EPR光谱,揭示了H 2 S选择性氧化的反应途径。进行密度泛函理论 (DFT) 计算以更深入地了解 Fe 掺杂对缺陷 TiO 2的电子结构和氧空位的影响。





























 京公网安备 11010802027423号
京公网安备 11010802027423号