当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ketone Enolization with Sodium Hexamethyldisilazide: Solvent- and Substrate-Dependent E–Z Selectivity and Affiliated Mechanisms
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-10-13 , DOI: 10.1021/jacs.1c06529 Ryan A Woltornist 1 , David B Collum 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-10-13 , DOI: 10.1021/jacs.1c06529 Ryan A Woltornist 1 , David B Collum 1
Affiliation
|
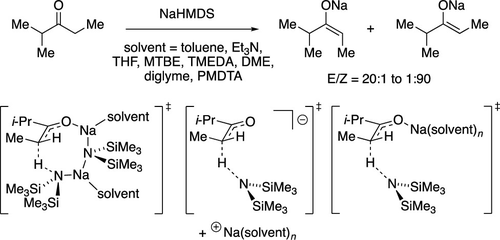
Ketone enolization by sodium hexamethyldisilazide (NaHMDS) shows a marked solvent and substrate dependence. Enolization of 2-methyl-3-pentanone reveals E–Z selectivities in Et3N/toluene (20:1), methyl-t-butyl ether (MTBE, 10:1), N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDTA)/toluene (8:1), TMEDA/toluene (4:1), diglyme (1:1), DME (1:22), and tetrahydrofuran (THF) (1:90). Control experiments show slow or nonexistent stereochemical equilibration in all solvents except THF. Enolate trapping with Me3SiCl/Et3N requires warming to −40 °C whereas Me3SiOTf reacts within seconds. In situ enolate trapping at −78 °C using preformed NaHMDS/Me3SiCl mixtures is effective in Et3N/toluene yet fails in THF by forming (Me3Si)3N. Rate studies show enolization via mono- and disolvated dimers in Et3N/toluene, disolvated dimers in TMEDA, trisolvated monomers in THF/toluene, and free ions with PMDTA. Density functional theory computations explore the selectivities via the E- and Z-based transition structures. Failures of theory-experiment correlations of ionic fragments were considerable even when isodesmic comparisons could have canceled electron correlation errors. Swapping 2-methyl-3-pentanone with a close isostere, 2-methylcyclohexanone, causes a fundamental change in the mechanism to a trisolvated-monomer-based enolization in THF.
中文翻译:

六甲基二硅氮化钠的酮烯醇化:溶剂和底物依赖性 E-Z 选择性和附属机制
六甲基二硅氮化钠 (NaHMDS) 的酮烯醇化表现出明显的溶剂和底物依赖性。 2-甲基-3-戊酮的烯醇化揭示了 Et 3 N/甲苯 (20:1)、甲基叔丁基醚 (MTBE, 10:1)、 N,N,N',N"中的E – Z选择性, N”-五甲基二亚乙基三胺 (PMDTA)/甲苯 (8:1)、TMEDA/甲苯 (4:1)、二甘醇二甲醚 (1:1)、DME (1:22) 和四氢呋喃 (THF) (1:90)。对照实验显示除 THF 之外的所有溶剂中立体化学平衡缓慢或不存在。使用 Me 3 SiCl/Et 3 N 捕获烯醇化物需要升温至 -40 °C,而 Me 3 SiOTf 在几秒钟内即可反应。使用预制的 NaHMDS/Me 3 SiCl 混合物在 -78 °C 下原位烯醇化物捕获在 Et 3 N/甲苯中有效,但在 THF 中则因形成 (Me 3 Si) 3 N 而失败。速率研究表明,通过单二聚体和溶解二聚体进行烯醇化Et 3 N/甲苯、TMEDA 中溶解的二聚体、THF/甲苯中的三溶剂化单体以及 PMDTA 中的游离离子。密度泛函理论计算通过基于E和Z的过渡结构探索选择性。即使等线比较可以消除电子相关误差,离子碎片的理论-实验相关性的失败也是相当大的。将 2-甲基-3-戊酮与接近的电子等排体 2-甲基环己酮交换,会导致 THF 中基于三溶剂化单体的烯醇化机制发生根本性变化。
更新日期:2021-10-27
中文翻译:

六甲基二硅氮化钠的酮烯醇化:溶剂和底物依赖性 E-Z 选择性和附属机制
六甲基二硅氮化钠 (NaHMDS) 的酮烯醇化表现出明显的溶剂和底物依赖性。 2-甲基-3-戊酮的烯醇化揭示了 Et 3 N/甲苯 (20:1)、甲基叔丁基醚 (MTBE, 10:1)、 N,N,N',N"中的E – Z选择性, N”-五甲基二亚乙基三胺 (PMDTA)/甲苯 (8:1)、TMEDA/甲苯 (4:1)、二甘醇二甲醚 (1:1)、DME (1:22) 和四氢呋喃 (THF) (1:90)。对照实验显示除 THF 之外的所有溶剂中立体化学平衡缓慢或不存在。使用 Me 3 SiCl/Et 3 N 捕获烯醇化物需要升温至 -40 °C,而 Me 3 SiOTf 在几秒钟内即可反应。使用预制的 NaHMDS/Me 3 SiCl 混合物在 -78 °C 下原位烯醇化物捕获在 Et 3 N/甲苯中有效,但在 THF 中则因形成 (Me 3 Si) 3 N 而失败。速率研究表明,通过单二聚体和溶解二聚体进行烯醇化Et 3 N/甲苯、TMEDA 中溶解的二聚体、THF/甲苯中的三溶剂化单体以及 PMDTA 中的游离离子。密度泛函理论计算通过基于E和Z的过渡结构探索选择性。即使等线比较可以消除电子相关误差,离子碎片的理论-实验相关性的失败也是相当大的。将 2-甲基-3-戊酮与接近的电子等排体 2-甲基环己酮交换,会导致 THF 中基于三溶剂化单体的烯醇化机制发生根本性变化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号