当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Synthesis of Taxol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-10-12 , DOI: 10.1021/jacs.1c09637 Ya-Jian Hu 1 , Chen-Chen Gu 1 , Xin-Feng Wang 1 , Long Min 1 , Chuang-Chuang Li 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-10-12 , DOI: 10.1021/jacs.1c09637 Ya-Jian Hu 1 , Chen-Chen Gu 1 , Xin-Feng Wang 1 , Long Min 1 , Chuang-Chuang Li 1
Affiliation
|
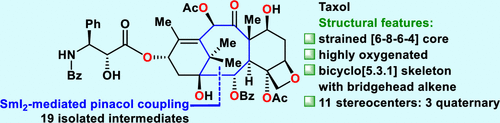
Taxol is one of the most famous natural diterpenoids and an important anticancer medicine. Taxol represents a formidable synthetic challenge and has prompted significant interest from the synthetic community. However, in all the previous syntheses of Taxol, there have been no reports of closing the desired eight-membered ring through C1–C2 bond formation. Furthermore, the existence of Taxol-resistant tumors and side effects of Taxol make the development of new approaches to synthesize Taxol and its derivatives highly desirable. Here, we report the asymmetric total synthesis of Taxol using a concise approach through 19 isolated intermediates. The synthetically challenging eight-membered ring was constructed efficiently by a diastereoselective intramolecular SmI2-mediated pinacol coupling reaction to form the C1–C2 bond. The unique biomimetic oxygen ene reaction and the newly developed facile tandem C2-benzoate formation and C13 side chain installation improved the efficiency of the synthesis. The mild oxygen ene reaction under light conditions would be an alternative reaction involved in Taxol biosynthesis. This new convergent approach will allow the diverse creation of Taxol derivatives to enable further biological research.
中文翻译:

紫杉醇的不对称全合成
紫杉醇是最著名的天然二萜类化合物之一,也是重要的抗癌药物。紫杉醇代表了一项艰巨的合成挑战,并引起了合成界的极大兴趣。然而,在以前的所有紫杉醇合成中,都没有关于通过 C1-C2 键形成闭合所需八元环的报道。此外,紫杉醇抗性肿瘤的存在和紫杉醇的副作用使得开发合成紫杉醇及其衍生物的新方法非常可取。在这里,我们使用简洁的方法通过 19 个分离的中间体报告了紫杉醇的不对称全合成。通过非对映选择性分子内 SmI 2有效构建了具有综合挑战性的八元环介导频哪醇偶联反应形成 C1-C2 键。独特的仿生氧烯反应和新开发的简便串联C2-苯甲酸酯形成和C13侧链安装提高了合成效率。光照条件下的温和氧烯反应将是涉及紫杉醇生物合成的替代反应。这种新的融合方法将允许紫杉醇衍生物的多样化创造,以实现进一步的生物学研究。
更新日期:2021-10-27
中文翻译:

紫杉醇的不对称全合成
紫杉醇是最著名的天然二萜类化合物之一,也是重要的抗癌药物。紫杉醇代表了一项艰巨的合成挑战,并引起了合成界的极大兴趣。然而,在以前的所有紫杉醇合成中,都没有关于通过 C1-C2 键形成闭合所需八元环的报道。此外,紫杉醇抗性肿瘤的存在和紫杉醇的副作用使得开发合成紫杉醇及其衍生物的新方法非常可取。在这里,我们使用简洁的方法通过 19 个分离的中间体报告了紫杉醇的不对称全合成。通过非对映选择性分子内 SmI 2有效构建了具有综合挑战性的八元环介导频哪醇偶联反应形成 C1-C2 键。独特的仿生氧烯反应和新开发的简便串联C2-苯甲酸酯形成和C13侧链安装提高了合成效率。光照条件下的温和氧烯反应将是涉及紫杉醇生物合成的替代反应。这种新的融合方法将允许紫杉醇衍生物的多样化创造,以实现进一步的生物学研究。







































 京公网安备 11010802027423号
京公网安备 11010802027423号