Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2021-10-13 , DOI: 10.1016/j.jtice.2021.09.029 Ying-Chou Su , Cheng-Chien Wang , Chuh-Yung Chen
|
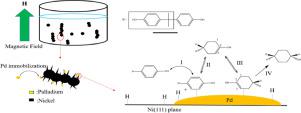
Background: 4,4′-isopropylidenediphenol (BPA) hydrogenated to 2,2-bis(4-hydroxycyclohexyl) propane (HBPA) is a simple way to reduce its toxicity and environmental impact. In addition, the higher trans/trans HBPA moiety in polymer will has better mechanical properties. Therefore, how to prepare trans/trans HBPA via hydrogenation is an important issue.
Method: One-dimensional nickel-palladium catalyst was successfully synthesized via reduction by an external magnetic field. The high ratio of trans/trans HBPA was characterized by FTIR, GC/MS, and NMR, respectively. The adsorption behavior of BPA and its half-hydrogenated intermediates was studied by Material Studio software.
Significant findings: The pd nanoparticles immobilized on the magnetization of 1-D Ni catalyst with high amount of Ni(111) plane is the novelty of this research. The conversion of BPA was high to 100% and the ratio of trans/trans HBPA was over than 75% at 180 °C and 70 kgf/cm2 in isopropanol medium. The trans/trans ratio of HBPA was slightly increasing as increasing hydrogen pressure and decreasing reaction temperature. Reaction intermediate was selected with the aid of molecular simulation. Moreover, the reaction path of BPA hydrogenated by one-dimensional nickel-palladium catalyst was proposed that BPA was firstly hydrogenated to tetrahydro intermediate and followed roll-over mechanism to obtain trans/trans HBPA product.
中文翻译:

一维镍钯催化剂制备高比反/反2,2-双(4-羟基环己基)丙烷异构体
背景:将 4,4'-异亚丙基二酚 (BPA) 氢化成 2,2-双(4-羟基环己基)丙烷 (HBPA) 是一种降低其毒性和环境影响的简单方法。此外,聚合物中较高的反式/反式 HBPA 部分将具有更好的机械性能。因此,如何通过加氢制备反式/反式HBPA是一个重要的问题。
方法:通过外磁场还原成功合成一维镍钯催化剂。反式/反式HBPA的高比率分别通过 FTIR、GC/MS 和 NMR 表征。通过Material Studio软件研究了BPA及其半氢化中间体的吸附行为。
重要发现:固定在具有大量 Ni(111) 面的一维 Ni 催化剂磁化上的 pd 纳米粒子是本研究的新颖之处。BPA 的转化率高达 100%,在 180 °C 和 70 kgf/cm 2的异丙醇介质中,反式/反式HBPA的比率超过 75% 。随着氢气压力的增加和反应温度的降低,HBPA的反式/反式比例略有增加。借助分子模拟选择反应中间体。此外,提出了一维镍钯催化剂加氢BPA的反应路径,先将BPA加氢为四氢中间体,然后按照翻转机制得到反式/反式。 HBPA 产品。






























 京公网安备 11010802027423号
京公网安备 11010802027423号