Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-10-13 , DOI: 10.1016/j.tetlet.2021.153467 Evgeny Chupakhin 1, 2 , Olga Bakulina 1 , Dmitry Dar'in 1 , Mikhail Krasavin 1, 2
|
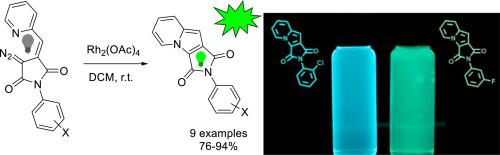
An unusual reactivity of Rh(II) carbenes generated from (E)-3-(2-pyridylmethylene)-4-diazopyrrolidine-2,5-diones was discovered which led to a novel type of fluorescent annelated indolizines (X-ray confirmed structure). The reaction was found to be insensitive to substitution pattern at pyrrolidine nitrogen atom and delivered a series of nine novel indolizines in 74–94% yields. Photophysical properties of obtained indolizines were investigated, which proved them to be blue or green luminophores with absolute quantum yields up to 13.8%. The latter strongly depended on substitution pattern at pyrrolidine nitrogen atom.
中文翻译:

通过分子内 Rh(II) 卡宾捕获轻松进入 1H-吡咯并 [3,4-b]indolizine-1,3(2H)-dione 支架
发现了由 ( E )-3-(2-吡啶基亚甲基)-4-diazopyrrolidine-2,5-diones产生的 Rh(II) 卡宾的不寻常反应性,这导致了一种新型的荧光退火 indolizines(X 射线证实结构)。发现该反应对吡咯烷氮原子的取代模式不敏感,并以 74-94% 的产率提供了一系列 9 种新型吲哚嗪。研究了获得的吲哚嗪的光物理性质,证明它们是蓝色或绿色发光体,绝对量子产率高达 13.8%。后者强烈依赖于吡咯烷氮原子的取代模式。






























 京公网安备 11010802027423号
京公网安备 11010802027423号