当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amyloid β Protein: Aβ40 Inhibits Aβ42 Oligomerization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-05-13 , DOI: 10.1021/ja8092604 Megan M Murray 1 , Summer L Bernstein , Vy Nyugen , Margaret M Condron , David B Teplow , Michael T Bowers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-05-13 , DOI: 10.1021/ja8092604 Megan M Murray 1 , Summer L Bernstein , Vy Nyugen , Margaret M Condron , David B Teplow , Michael T Bowers
Affiliation
|
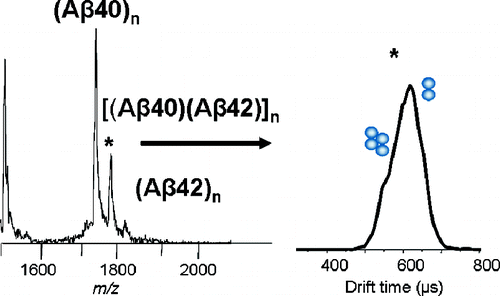
Abeta40 and Abeta42 are peptides that adopt similar random-coil structures in solution. Abeta42, however, is significantly more neurotoxic than Abeta40 and forms amyloid fibrils much more rapidly than Abeta40. Here, mass spectrometry and ion mobility spectrometry are used to investigate a mixture of Abeta40 and Abeta42. The mass spectrum for the mixed solution shows the presence of a heterooligomer composed of equal parts of Abeta40 and Abeta42. Ion mobility results indicate that this mixed species comprises an oligomer distribution extending to tetramers. Abeta40 alone produces such a distribution, whereas Abeta42 alone produces oligomers as large as dodecamers. This indicates that Abeta40 inhibits Abeta42 oligomerization.
中文翻译:

β 淀粉样蛋白:Aβ40 抑制 Aβ42 寡聚化
Abeta40 和 Abeta42 是在溶液中采用类似随机螺旋结构的肽。然而,Abeta42 的神经毒性显着高于 Abeta40,并且形成淀粉样原纤维的速度比 Abeta40 快得多。在这里,使用质谱法和离子迁移谱法来研究 Abeta40 和 Abeta42 的混合物。混合溶液的质谱显示存在由等份的Abeta40和Abeta42组成的杂低聚物。离子淌度结果表明该混合物质包含延伸至四聚体的低聚物分布。 Abeta40单独产生这样的分布,而Abeta42单独产生与十二聚体一样大的寡聚体。这表明Abeta40抑制Abeta42寡聚化。
更新日期:2009-05-13
中文翻译:

β 淀粉样蛋白:Aβ40 抑制 Aβ42 寡聚化
Abeta40 和 Abeta42 是在溶液中采用类似随机螺旋结构的肽。然而,Abeta42 的神经毒性显着高于 Abeta40,并且形成淀粉样原纤维的速度比 Abeta40 快得多。在这里,使用质谱法和离子迁移谱法来研究 Abeta40 和 Abeta42 的混合物。混合溶液的质谱显示存在由等份的Abeta40和Abeta42组成的杂低聚物。离子淌度结果表明该混合物质包含延伸至四聚体的低聚物分布。 Abeta40单独产生这样的分布,而Abeta42单独产生与十二聚体一样大的寡聚体。这表明Abeta40抑制Abeta42寡聚化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号