Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-10-12 , DOI: 10.1016/j.bioorg.2021.105423 Yue Li 1 , Jin-He Zhang 1 , Hong-Xu Xie 1 , Yong-Xi Ge 1 , Kai-Ming Wang 1 , Zhi-Ling Song 2 , Kong-Kai Zhu 3 , Juan Zhang 1 , Cheng-Shi Jiang 1
|
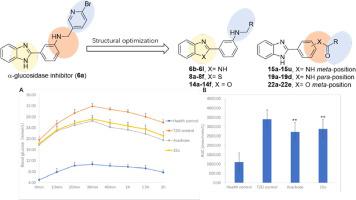
In the present study, a series of 2-phenyl-1H-benzo[d]imidazole-based α-glucosidase inhibitors were synthesized and evaluated for their in vitro and in vivo anti-diabetic potential. Screening of an in-house library revealed a moderated α-glucosidase inhibitor, 6a with 3-(1H-benzo[d]imidazol-2-yl)aniline core, and then the structural optimization was performed to obtain more efficient derivatives. Most of these derivatives showed increased activity than 6a, and the most promising inhibitors were found to be compounds 15o and 22d with IC50 values of 2.09 ± 0.04 and 0.71 ± 0.02 µM, respectively. Fluorescence quenching experiment confirmed the direct binding of compounds 15o and 22d with α-glucosidase. Kinetic study revealed that both compounds were non-competitive inhibitors, that was consistent with the result of molecular docking studies where they located at the allosteric site of the enzyme. Cell viability evaluation demonstrated the non-cytotoxicity of 15o and 22d against LO2 cells. Furthermore, the in vivo pharmacodynamic study revealed that compound 15o showed significant hypoglycemic activity and improved oral sucrose tolerance, comparable to the positive control acarbose.
中文翻译:

发现新的基于 2-苯基-1H-苯并[d]咪唑核心的强效 α-葡萄糖苷酶抑制剂:合成、动力学研究、分子对接和体内抗高血糖评估
在本研究中,合成了一系列基于 2-苯基-1 H-苯并[ d ]咪唑的 α-葡萄糖苷酶抑制剂,并评估了它们的体外和体内抗糖尿病潜力。对内部库的筛选揭示了一种缓和的 α-葡萄糖苷酶抑制剂,具有 3-(1 H-苯并[ d ]咪唑-2-基)苯胺核心的6a ,然后进行结构优化以获得更有效的衍生物。大多数这些衍生物显示出比6a更高的活性,发现最有希望的抑制剂是化合物15o和22d,IC 50值分别为 2.09 ± 0.04 和 0.71 ± 0.02 µM。荧光猝灭实验证实了化合物15o和22d与 α-葡萄糖苷酶的直接结合。动力学研究表明,这两种化合物都是非竞争性抑制剂,这与分子对接研究的结果一致,它们位于酶的变构位点。细胞活力评估证明了15o和22d对 LO2 细胞的非细胞毒性。此外,体内药效学研究表明,与阳性对照阿卡波糖相比,化合物15o显示出显着的降血糖活性和改善的口服蔗糖耐受性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号