当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonadiabatic Effects in the Molecular Oxidation of Subnanometric Cu5 Clusters
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-10-11 , DOI: 10.1021/acs.jpca.1c07271
Alexander O Mitrushchenkov 1 , Alexandre Zanchet 2 , Andreas W Hauser 3 , María Pilar de Lara-Castells 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-10-11 , DOI: 10.1021/acs.jpca.1c07271
Alexander O Mitrushchenkov 1 , Alexandre Zanchet 2 , Andreas W Hauser 3 , María Pilar de Lara-Castells 2
Affiliation
|
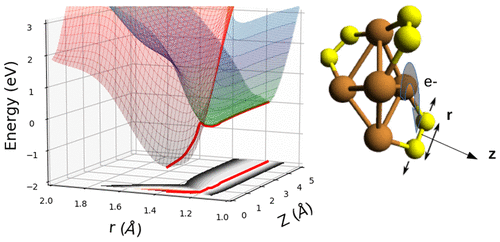
The electronic structure of subnanometric clusters, far off the bulk regime, is still dominated by molecular characteristics. The spatial arrangement of the notoriously undercoordinated metal atoms is strongly coupled to the electronic properties of the system, which makes this class of materials particularly interesting for applications including luminescence, sensing, bioimaging, theranostics, energy conversion, catalysis, and photocatalysis. Opposing a common rule of thumb that assumes an increasing chemical reactivity with smaller cluster size, Cu5 clusters have proven to be exceptionally resistant to irreversible oxidation, i.e., the dissociative chemisorption of molecular oxygen. Besides providing reasons for this behavior in the case of heavy loading with molecular oxygen, we investigate the competition between physisorption and molecular chemisorption from the perspective of nonadiabatic effects. Landau–Zener theory is applied to the Cu5(O2)3 complex to estimate the probability for a switching between the electronic states correlating the neutral O2 + Cu5(O2)2 and the ionic O2– + (Cu5(O2)2)+ fragments in a diabatic representation. Our work demonstrates the involvement of strong nonadiabatic effects in the associated charge transfer process, which might be a common motive in reactions involving subnanometric metal structures.
中文翻译:

亚纳米Cu5簇分子氧化中的非绝热效应
亚纳米簇的电子结构,远离体态,仍然由分子特征主导。众所周知的配位不足的金属原子的空间排列与系统的电子特性强耦合,这使得这类材料在发光、传感、生物成像、治疗诊断学、能量转换、催化和光催化等应用中特别有趣。反对一个普遍的经验法则,即假设随着较小的簇大小,Cu 5 的化学反应性增加团簇已被证明对不可逆氧化(即分子氧的解离化学吸附)具有非凡的抵抗力。除了在分子氧重载的情况下提供这种行为的原因外,我们还从非绝热效应的角度研究了物理吸附和分子化学吸附之间的竞争。Landau-Zener 理论应用于 Cu 5 (O 2 ) 3 配合物,以估计与中性 O 2 + Cu 5 (O 2 ) 2和离子 O 2 – + (Cu 5 (O 2 ) 2) +非绝热表示中的片段。我们的工作证明了强非绝热效应参与相关的电荷转移过程,这可能是涉及亚纳米金属结构的反应的常见动机。
更新日期:2021-10-22
中文翻译:

亚纳米Cu5簇分子氧化中的非绝热效应
亚纳米簇的电子结构,远离体态,仍然由分子特征主导。众所周知的配位不足的金属原子的空间排列与系统的电子特性强耦合,这使得这类材料在发光、传感、生物成像、治疗诊断学、能量转换、催化和光催化等应用中特别有趣。反对一个普遍的经验法则,即假设随着较小的簇大小,Cu 5 的化学反应性增加团簇已被证明对不可逆氧化(即分子氧的解离化学吸附)具有非凡的抵抗力。除了在分子氧重载的情况下提供这种行为的原因外,我们还从非绝热效应的角度研究了物理吸附和分子化学吸附之间的竞争。Landau-Zener 理论应用于 Cu 5 (O 2 ) 3 配合物,以估计与中性 O 2 + Cu 5 (O 2 ) 2和离子 O 2 – + (Cu 5 (O 2 ) 2) +非绝热表示中的片段。我们的工作证明了强非绝热效应参与相关的电荷转移过程,这可能是涉及亚纳米金属结构的反应的常见动机。

































 京公网安备 11010802027423号
京公网安备 11010802027423号