当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spatial Location and Microenvironment Engineering of Pt-CeO2 Nanoreactors for Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-10-10 , DOI: 10.1021/acs.jpcc.1c07693 Liwei Wang 1, 2 , Rongrong Han 1, 2 , Yanfu Ma 1 , Melis S. Duyar 3, 4 , Wei Liu 1 , Jian Liu 1, 2, 5
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-10-10 , DOI: 10.1021/acs.jpcc.1c07693 Liwei Wang 1, 2 , Rongrong Han 1, 2 , Yanfu Ma 1 , Melis S. Duyar 3, 4 , Wei Liu 1 , Jian Liu 1, 2, 5
Affiliation
|
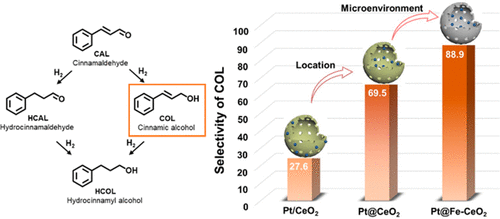
More than 25% of chemical transformations involve at least one hydrogenation step. Selective hydrogenation of unsaturated aldehydes is an essential process in the industrial production of pesticides and pharmaceutical synthesis. Since C═C hydrogenation with lower bond energy is thermodynamically favored over C═O hydrogenation, the selective hydrogenation of cinnamaldehyde (CAL) to cinnamyl alcohol (COL) is relatively challenging. Herein, we report a series of Pt-CeO2 nanoreactors with different spatial locations and microenvironments of Pt nanoparticles (NPs) on hollow CeO2 that are active for the selective hydrogenation of CAL to COL. We show the effects of active metal spatial location, microenvironment, metal–support interactions, and Fe doping on the activity and selectivity within Pt-CeO2 nanoreactors. Pt@Fe-CeO2 shows excellent catalytic performance with an 88.9% selectivity for COL at a CAL conversion of 97.2%. The variations of the electronic and crystal structure after Fe doping, simultaneously, and the linear adsorption of CAL on the CeO2 hollow structure contribute to the high performance of selective hydrogenation to COL. Our findings might shed light on the rational design of the nanoreactors for catalytic organic transformations with desired selectivity.
中文翻译:

用于肉桂醛选择性加氢制肉桂醇的 Pt-CeO2 纳米反应器的空间定位和微环境工程
超过 25% 的化学转化涉及至少一个氢化步骤。不饱和醛的选择性加氢是农药工业生产和药物合成中必不可少的过程。由于具有较低键能的 C=C 氢化在热力学上优于 C=O 氢化,因此肉桂醛 (CAL) 选择性氢化成肉桂醇 (COL) 相对具有挑战性。在此,我们报告了一系列 Pt-CeO 2纳米反应器,在空心 CeO 2上具有不同空间位置和 Pt 纳米粒子 (NPs) 的微环境对 CAL 选择性氢化为 COL 具有活性。我们展示了活性金属空间位置、微环境、金属-载体相互作用和 Fe 掺杂对 Pt-CeO 2纳米反应器内活性和选择性的影响。Pt@Fe-CeO 2表现出优异的催化性能,对 COL 的选择性为 88.9%,CAL 转化率为 97.2%。Fe掺杂后电子和晶体结构的变化,同时CAL在CeO 2空心结构上的线性吸附有助于选择性氢化成COL的高性能。我们的研究结果可能会阐明用于具有所需选择性的催化有机转化的纳米反应器的合理设计。
更新日期:2021-10-22
中文翻译:

用于肉桂醛选择性加氢制肉桂醇的 Pt-CeO2 纳米反应器的空间定位和微环境工程
超过 25% 的化学转化涉及至少一个氢化步骤。不饱和醛的选择性加氢是农药工业生产和药物合成中必不可少的过程。由于具有较低键能的 C=C 氢化在热力学上优于 C=O 氢化,因此肉桂醛 (CAL) 选择性氢化成肉桂醇 (COL) 相对具有挑战性。在此,我们报告了一系列 Pt-CeO 2纳米反应器,在空心 CeO 2上具有不同空间位置和 Pt 纳米粒子 (NPs) 的微环境对 CAL 选择性氢化为 COL 具有活性。我们展示了活性金属空间位置、微环境、金属-载体相互作用和 Fe 掺杂对 Pt-CeO 2纳米反应器内活性和选择性的影响。Pt@Fe-CeO 2表现出优异的催化性能,对 COL 的选择性为 88.9%,CAL 转化率为 97.2%。Fe掺杂后电子和晶体结构的变化,同时CAL在CeO 2空心结构上的线性吸附有助于选择性氢化成COL的高性能。我们的研究结果可能会阐明用于具有所需选择性的催化有机转化的纳米反应器的合理设计。






































 京公网安备 11010802027423号
京公网安备 11010802027423号