当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antiaromaticity-Promoted Radical Stability in α-Methyl Heterocyclics
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-10-11 , DOI: 10.1021/acs.joc.1c02050 Lu Lin 1 , Jun Zhu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-10-11 , DOI: 10.1021/acs.joc.1c02050 Lu Lin 1 , Jun Zhu 1
Affiliation
|
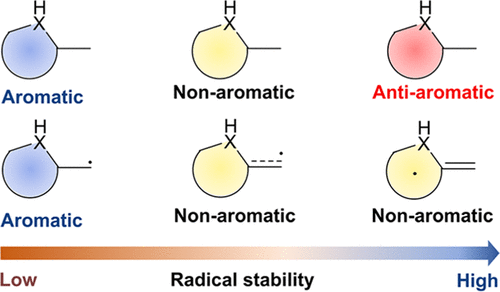
Aromaticity is a fundamental and important concept in chemistry, and usually, the enhancement of aromaticity brings additional thermodynamic stability to a compound. Moreover, since radicals can act as intermediates in chemical reactions, they have attracted considerable attention from both experimental and theoretical chemists for a long time. However, it remains unclear whether there is a relationship between the thermodynamic stability of cyclic planar radicals and their aromaticity. In this work, using various aromaticity indices including anisotropy of the induced current density analysis and nucleus-independent chemical shifts against the radical stabilization energy, we systematically investigated the relationship between aromaticity and the thermodynamic stability of α-methyl heterocyclics. Density functional theory calculations suggest that the stronger the antiaromaticity of the original form heterocyclics, the higher the thermodynamic stability of the corresponding radicals, which is in sharp contrast to the general knowledge that aromaticity brings compounds’ thermodynamic stabilities. The principal interacting spin orbital analysis shows that the stronger the π-bond formed between the heterocyclics and the α-methyl carbon, the more spin density the radicals tend to be distributed on the heterocyclics. Thus, the strong π-bonding is one of the factors for improving the thermodynamic stability of radicals.
中文翻译:

α-甲基杂环化合物中抗芳香性促进的自由基稳定性
芳香性是化学中一个基本且重要的概念,通常,芳香性的增强会给化合物带来额外的热力学稳定性。此外,由于自由基可以作为化学反应的中间体,长期以来一直受到实验和理论化学家的广泛关注。然而,环状平面自由基的热力学稳定性与其芳香性之间是否存在关系仍不清楚。在这项工作中,我们利用各种芳香性指数,包括感应电流密度分析的各向异性和与自由基稳定能的核无关化学位移,系统地研究了芳香性与 α-甲基杂环的热力学稳定性之间的关系。密度泛函理论计算表明,原始形式杂环的反芳香性越强,相应自由基的热力学稳定性越高,这与芳香性带来化合物热力学稳定性的一般知识形成鲜明对比。主要的相互作用自旋轨道分析表明,杂环与α-甲基碳之间形成的π键越强,自由基倾向于分布在杂环上的自旋密度越大。因此,强π键是提高自由基热力学稳定性的因素之一。这与芳香性带来化合物热力学稳定性的常识形成鲜明对比。主要相互作用自旋轨道分析表明,杂环与α-甲基碳之间形成的π键越强,自由基倾向于分布在杂环上的自旋密度越大。因此,强π键是提高自由基热力学稳定性的因素之一。这与芳香性带来化合物热力学稳定性的常识形成鲜明对比。主要的相互作用自旋轨道分析表明,杂环与α-甲基碳之间形成的π键越强,自由基倾向于分布在杂环上的自旋密度越大。因此,强π键是提高自由基热力学稳定性的因素之一。
更新日期:2021-11-05
中文翻译:

α-甲基杂环化合物中抗芳香性促进的自由基稳定性
芳香性是化学中一个基本且重要的概念,通常,芳香性的增强会给化合物带来额外的热力学稳定性。此外,由于自由基可以作为化学反应的中间体,长期以来一直受到实验和理论化学家的广泛关注。然而,环状平面自由基的热力学稳定性与其芳香性之间是否存在关系仍不清楚。在这项工作中,我们利用各种芳香性指数,包括感应电流密度分析的各向异性和与自由基稳定能的核无关化学位移,系统地研究了芳香性与 α-甲基杂环的热力学稳定性之间的关系。密度泛函理论计算表明,原始形式杂环的反芳香性越强,相应自由基的热力学稳定性越高,这与芳香性带来化合物热力学稳定性的一般知识形成鲜明对比。主要的相互作用自旋轨道分析表明,杂环与α-甲基碳之间形成的π键越强,自由基倾向于分布在杂环上的自旋密度越大。因此,强π键是提高自由基热力学稳定性的因素之一。这与芳香性带来化合物热力学稳定性的常识形成鲜明对比。主要相互作用自旋轨道分析表明,杂环与α-甲基碳之间形成的π键越强,自由基倾向于分布在杂环上的自旋密度越大。因此,强π键是提高自由基热力学稳定性的因素之一。这与芳香性带来化合物热力学稳定性的常识形成鲜明对比。主要的相互作用自旋轨道分析表明,杂环与α-甲基碳之间形成的π键越强,自由基倾向于分布在杂环上的自旋密度越大。因此,强π键是提高自由基热力学稳定性的因素之一。































 京公网安备 11010802027423号
京公网安备 11010802027423号