Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-10-09 , DOI: 10.1016/j.bioorg.2021.105408 Javeed Ur Rasool 1 , Gifty Sawhney 2 , Majeed Shaikh 3 , Yedukondalu Nalli 3 , Sreedhar Madishetti 2 , Zabeer Ahmed 2 , Asif Ali 4
|
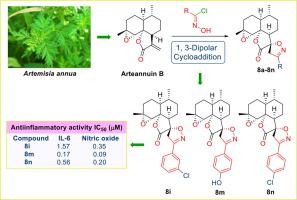
A library of new spiroisoxazoline analogues of arteannuin B was synthesized through 1, 3-dipolar cycloaddition in stereoselective fashion and consequently screened for anti-inflammatory activity in RAW 264.7 macrophage cells. Three potent analogues (8i, 8 m, and 8n) were found to attenuate the LPS induced release of cytokines IL-6 and TNF-α more potently than the parent molecule. Also, the inhibition of LPS induced nitric oxide production in these cells show moderate to high efficacy. None of the three potent molecules have altered the viability of RAW 264.7 cells following 48 h incubation suggesting that the inhibition of cytokines and nitric oxide production exhibited in the cells was not due to toxicity. In addition, these compounds exhibit an IC50 range of 0.17 µM-1.57 µM and 0.09 µM-0.35 µM for the inhibition of IL-6 release and nitric oxide production respectively. The results disclose potent inhibition of pro-inflammatory mediators which are encouraging and warrant further investigations to develop new therapeutic agents for inflammatory diseases.
中文翻译:

青蒿素 B 螺异恶唑啉缝合加合物的定点合成及抗炎评价
青蒿素 B 的新螺异恶唑啉类似物文库通过 1, 3-偶极环加成以立体选择性方式合成,因此筛选 RAW 264.7 巨噬细胞的抗炎活性。发现三种有效的类似物(8i、8m和8n)比母体分子更有效地减弱 LPS 诱导的细胞因子 IL-6 和 TNF-α 的释放。此外,在这些细胞中对 LPS 诱导的一氧化氮产生的抑制显示出中等至高效的功效。在孵育 48 小时后,这三种有效分子均未改变 RAW 264.7 细胞的活力,这表明细胞中表现出的细胞因子和一氧化氮产生的抑制不是由于毒性。此外,这些化合物的 IC 50范围为 0.17 µM-1.57 µM 和 0.09 µM-0.35 µM,分别用于抑制 IL-6 释放和一氧化氮产生。结果揭示了对促炎介质的有效抑制,这是令人鼓舞的并且值得进一步研究以开发用于炎性疾病的新治疗剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号