Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2021-10-08 , DOI: 10.1016/j.bmcl.2021.128413 Jin-He Zhang 1 , Hong-Xu Xie 1 , Yue Li 1 , Kai-Ming Wang 1 , Zhiling Song 2 , Kong-Kai Zhu 3 , Lei Fang 1 , Juan Zhang 1 , Cheng-Shi Jiang 1
|
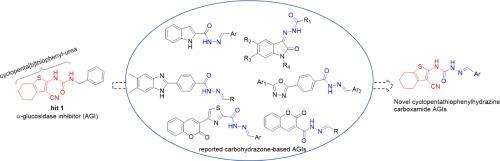
In this present study, a series of novel (E)-2-benzylidene-N-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)hydrazine-1-carboxamide derivatives against α-glucosidase were designed and synthesized, and their biological activities were evaluated in vitro and in vivo. Most of the designed analogues exhibited better inhibitory activity than the marketed acarbose, especially the most potent compound 7 with an IC50 value of 9.26 ± 1.84 μM. The direct binding of 7 and 8 with α-glucosidase was confirmed by fluorescence quenching experiments, and the kinetic and molecular docking studies revealed that 7 and 8 inhibited α-glucosidase in a non-competitive manner. Cytotoxicity bioassay indicated compounds 7 and 8 were non-toxic towards LO2 and HepG2 at 100 μM. Furthermore, both compounds were demonstrated to have in vivo hypoglycemic activity by reducing the blood glucose levels in sucrose-treated rats.
中文翻译:

新型 (E)-2-benzylidene-N-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)hydrazine-1-carboxamide 衍生物作为 α- 的设计、合成和生物学评价葡萄糖苷酶抑制剂
在此研究中,一系列新(的ë)-2-苄基- ñ - (3-氰基-4,5,6,7-四氢苯并[ b ]噻吩-2-基)抗α肼-1-甲酰胺衍生物设计并合成了β-葡萄糖苷酶,并在体外和体内对其生物学活性进行了评价。大多数设计的类似物比市售的阿卡波糖表现出更好的抑制活性,尤其是最有效的化合物 7,IC 为50值为 9.26 ± 1.84 μM。荧光猝灭实验证实了7和8与α-葡萄糖苷酶的直接结合,动力学和分子对接研究表明7和8以非竞争性方式抑制α-葡萄糖苷酶。细胞毒性生物测定表明化合物 7 和 8 在 100 μM 时对 LO2 和 HepG2 无毒。此外,通过降低蔗糖处理的大鼠的血糖水平,两种化合物都被证明具有体内降血糖活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号