当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Double Capture of Difluorocarbene by 2-Aminostyrenes Enables the Construction of 3-(2,2-Difluoroethyl)-2-fluoroindoles
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-07 , DOI: 10.1021/acs.orglett.1c02816 Heyun Sheng 1 , Jianke Su 1 , Xin Li 1 , Xue Li 1 , Qiuling Song 1, 2, 3
Organic Letters ( IF 4.9 ) Pub Date : 2021-10-07 , DOI: 10.1021/acs.orglett.1c02816 Heyun Sheng 1 , Jianke Su 1 , Xin Li 1 , Xue Li 1 , Qiuling Song 1, 2, 3
Affiliation
|
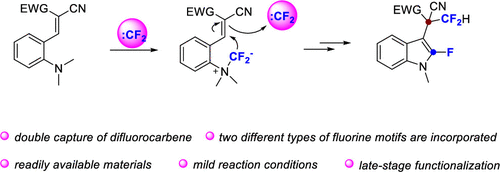
We report herein an efficient strategy to construct 3-(2,2-difluoroethyl)-2-fluoroindoles from activated o-aminostyrenes with ethyl bromodi-fluoroacetate as a difluorocarbene source. Through double capture of a difluorocarbene, two different types of fluorine motifs are incorporated into the products with simultaneous construction of one C–N and two C–C bonds, without the need for transition metals. This reaction features high efficiency and excellent functional group compatibility and has great potential in the late-stage modifications of pharmaceutical molecules and natural products.
中文翻译:

2-氨基苯乙烯对二氟卡宾的双重捕获使构建3-(2,2-二氟乙基)-2-氟吲哚成为可能
我们在此报告了一种从活化的邻氨基苯乙烯以溴二氟乙酸乙酯作为二氟卡宾来源构建 3-(2,2-二氟乙基)-2-氟吲哚的有效策略。通过双氟卡宾的双重捕获,两种不同类型的氟基元被结合到产品中,同时构建一个 C-N 键和两个 C-C 键,无需过渡金属。该反应具有高效和优异的官能团相容性,在药物分子和天然产物的后期修饰方面具有巨大潜力。
更新日期:2021-10-15
中文翻译:

2-氨基苯乙烯对二氟卡宾的双重捕获使构建3-(2,2-二氟乙基)-2-氟吲哚成为可能
我们在此报告了一种从活化的邻氨基苯乙烯以溴二氟乙酸乙酯作为二氟卡宾来源构建 3-(2,2-二氟乙基)-2-氟吲哚的有效策略。通过双氟卡宾的双重捕获,两种不同类型的氟基元被结合到产品中,同时构建一个 C-N 键和两个 C-C 键,无需过渡金属。该反应具有高效和优异的官能团相容性,在药物分子和天然产物的后期修饰方面具有巨大潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号