当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective [4 + 3] annulation of azadienes and ethyl 4-bromo-3-oxobutanoate: construction of benzindeno-fused azepine derivatives
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-29 , DOI: 10.1039/d1ob01749g Jinhui Shen 1 , Aimin Yu 1 , Xiangtai Meng 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-29 , DOI: 10.1039/d1ob01749g Jinhui Shen 1 , Aimin Yu 1 , Xiangtai Meng 1
Affiliation
|
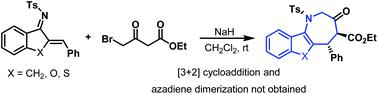
The benzindenoazepine ring system is an attractive scaffold for biologically active compounds. This work reported a NaH-promoted cycloaddition between azadienes and ethyl 4-bromo-3-oxobutanoate, which delivered a series of benzindenoazepines with good yields and stereoselectivities. Such benzindenoazepine derivatives were not easily obtained by using a traditional approach. The application of this cycloaddition strategy has been extended to azadienes bearing a benzofuran or benzothiophene moiety. The utility of this method was showcased by gram-scale experiments and synthetic transformations of the product.
中文翻译:

氮杂二烯和 4-溴-3-氧代丁酸乙酯的立体选择性 [4 + 3] 环化:苯并芘稠合氮杂衍生物的构建
苯并二氮杂环系统是一种有吸引力的生物活性化合物支架。这项工作报道了 NaH 促进的氮杂二烯和 4-溴-3-氧代丁酸乙酯之间的环加成反应,产生了一系列具有良好收率和立体选择性的苯并二氮杂卓。使用传统方法不容易获得此类苯并二氮杂卓衍生物。这种环加成策略的应用已扩展到带有苯并呋喃或苯并噻吩部分的氮杂二烯。通过克级实验和产品的合成转化展示了该方法的实用性。
更新日期:2021-10-07
中文翻译:

氮杂二烯和 4-溴-3-氧代丁酸乙酯的立体选择性 [4 + 3] 环化:苯并芘稠合氮杂衍生物的构建
苯并二氮杂环系统是一种有吸引力的生物活性化合物支架。这项工作报道了 NaH 促进的氮杂二烯和 4-溴-3-氧代丁酸乙酯之间的环加成反应,产生了一系列具有良好收率和立体选择性的苯并二氮杂卓。使用传统方法不容易获得此类苯并二氮杂卓衍生物。这种环加成策略的应用已扩展到带有苯并呋喃或苯并噻吩部分的氮杂二烯。通过克级实验和产品的合成转化展示了该方法的实用性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号