Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2021-10-05 , DOI: 10.1016/j.reactfunctpolym.2021.105062 Masoud Faal 1 , Mojtaba Mahyari 1 , Seyed Ghorban Hosseini 1 , Saeed Tavangar 1 , Mohammad Ali Zarei 1
|
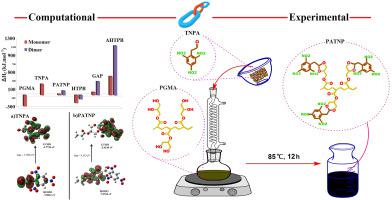
In recent years, acrylate binders have been widely used in PBX production. High loading of explosives and processability are the prominent features of these binders that researchers are trying to improve the energetic properties of these binders. In this research, a novel energetic binder of poly (2,4,6-trinitrophenylacetal acrylate) (PATNP) was synthesized through acetalization reaction Poly(glycerol monoacrylate) with 2,4,6-trinitrophenyl acetaldehyde in the presence of p-toluene sulfonic acid (PTSA) as the catalyst. The polymer structure was confirmed using 1H NMR and FT-IR. Thermal analysis obtained from the TGA curve of polymer shows that the addition of TNT derivatives significantly changes the thermal decomposition processes. In the TGA curve of PATNP, two main weight losses have been observed with a decomposition percentage of 38 and 37%. The glass transition temperatures (Tg) of poly(glycerol monoacrylate) and PATNP were measured by DSC, and their Tg were obtained at −51 °C and − 43 °C, respectively. The heat of formation (HOF) and the HOMO-LUMO gap of PATNP was calculated using DFT computational method and compared with HTPB, AHTPB, and GAP binders. The HOF of PATNP for the monomer and dimer was obtained as 70.67 and 263.26 J/g, respectively. The HOMO-LUMO gap for TNPA and PATNP was calculated at 3.7296 and 4.152 eV, respectively. The obtained energetic polymer can have a potential application as the binder of PBXs.
中文翻译:

聚(2,4,6-三硝基苯乙缩醛丙烯酸酯)新型含能粘合剂的合成及其生成热计算:理论与实验研究
近年来,丙烯酸酯粘合剂在PBX生产中得到了广泛的应用。炸药的高载量和可加工性是这些粘合剂的突出特点,研究人员正试图改善这些粘合剂的能量特性。在这项研究中,通过聚(甘油单丙烯酸酯)与 2,4,6-三硝基苯乙醛在对甲苯磺酸存在下的缩醛化反应合成了一种新型的聚(2,4,6-三硝基苯缩醛丙烯酸酯)(PATNP)的含能粘合剂。酸(PTSA)作为催化剂。使用1H NMR 和 FT-IR。从聚合物的 TGA 曲线获得的热分析表明,TNT 衍生物的加入显着改变了热分解过程。在 PATNP 的 TGA 曲线中,观察到了两个主要的重量损失,分解百分比分别为 38% 和 37%。聚(甘油单丙烯酸酯)和 PATNP的玻璃化转变温度 (T g ) 通过 DSC 测量,它们的 T g分别在 -51 °C 和 - 43 °C 下获得。使用 DFT 计算方法计算 PATNP 的形成热 (HOF) 和 HOMO-LUMO 间隙,并与 HTPB、AHTPB 和 GAP 粘合剂进行比较。单体和二聚体的 PATNP 的 HOF 分别为 70.67 和 263.26 J/g,分别。TNPA 和 PATNP 的 HOMO-LUMO 间隙分别计算为 3.7296 和 4.152 eV。所获得的含能聚合物作为 PBX 的粘合剂具有潜在的应用价值。































 京公网安备 11010802027423号
京公网安备 11010802027423号