客观的
长效胰高血糖素样肽 1 受体激动剂 (GLP-1RA),如利拉鲁肽和索马鲁肽,是治疗糖尿病和肥胖症的可行方法。利拉鲁肽在体外直接激活下丘脑阿片黑皮质素原 (POMC) 神经元,同时间接抑制神经肽 Y/Agouti 相关肽 (NPY/AgRP) 神经元。虽然在体外可以相对容易地实现 GLP-1R 激动剂浓度的时间控制以及对组织/细胞的可及性,但在体内这取决于这些激动剂的药代动力学和对感兴趣结构的相对渗透。因此,利拉鲁肽或索马鲁肽是否会改变体内POMC 和 NPY/AgRP 神经元的活性以及细胞活性发生任何变化所需的机制仍不清楚。
方法
为了解决这个问题,我们利用神经元特异性转基因小鼠模型来检查注射利拉鲁肽或索马鲁肽(腹膜内 - IP 和皮下 - S·C)后 POMC 和 NPY/AgRP 神经元活性的变化。POMC 和 NPY/AgRP 神经元被用于膜片钳电生理学以及体内光纤光度测定。
结果
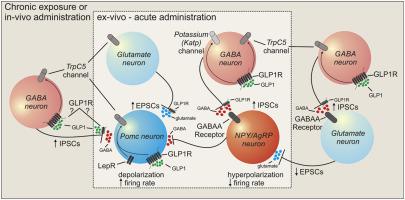
我们发现利拉鲁肽和索马鲁肽以时间依赖性方式直接激活并增加 POMC 神经元的兴奋性张力。POMC 神经元活性的增加需要 POMC 神经元中的 GLP-1R 以及由 TRPC5 亚基组成的下游混合阳离子通道。我们还观察到源自也需要 TRPC5 亚基的谷氨酸能细胞的 POMC 神经元兴奋性输入的间接上调。相反,GLP-1Ra 通过激活 GABA 能神经元中的 K-ATP 和 TRPC5 通道,减少对 NPY/AgRP 神经元的兴奋性输入并间接抑制。值得注意的是,注射利拉鲁肽或司马鲁肽后,POMC 的暂时激活和 NPY/AgRP 神经元活性的抑制[腹膜内 (IP) 或皮下 (S·C.)] 取决于动物的营养状态(喂养与食物-被剥夺)。
结论
我们的结果支持利拉鲁肽和索马鲁肽在体内激活 POMC 同时抑制 NPY/AgRP 神经元的机制,该机制取决于代谢状态并反映了这些化合物在体内的药代动力学特征。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo
Objective
Long-acting glucagon-like peptide-1 receptor agonists (GLP-1RAs), like liraglutide and semaglutide, are viable treatments for diabetes and obesity. Liraglutide directly activates hypothalamic proopiomelanocortin (POMC) neurons while indirectly inhibiting Neuropeptide Y/Agouti-related peptide (NPY/AgRP) neurons ex vivo. While temporal control of GLP-1R agonist concentration as well as accessibility to tissues/cells can be achieved with relative ease ex vivo, in vivo this is dependent upon the pharmacokinetics of these agonists and relative penetration into structures of interest. Thus, whether liraglutide or semaglutide modifies the activity of POMC and NPY/AgRP neurons in vivo as well as mechanisms required for any changes in cellular activity remains undefined.
Methods
In order to resolve this issue, we utilized neuron-specific transgenic mouse models to examine changes in the activity of POMC and NPY/AgRP neurons after injection of either liraglutide or semaglutide (intraperitoneal - I.P. and subcutaneous - S·C.). POMC and NPY/AgRP neurons were targeted for patch-clamp electrophysiology as well as in vivo fiber photometry.
Results
We found that liraglutide and semaglutide directly activate and increase excitatory tone to POMC neurons in a time-dependent manner. This increased activity of POMC neurons required GLP-1Rs in POMC neurons as well as a downstream mixed cation channel comprised of TRPC5 subunits. We also observed an indirect upregulation of excitatory input to POMC neurons originating from glutamatergic cells that also required TRPC5 subunits. Conversely, GLP-1Ra's decreased excitatory input to and indirectly inhibited NPY/AgRP neurons through activation of K-ATP and TRPC5 channels in GABAergic neurons. Notably, the temporal activation of POMC and inhibition of NPY/AgRP neuronal activity after liraglutide or semaglutide was injected [either intraperitoneal (I.P.) or subcutaneous (S·C.)] was dependent upon the nutritional state of the animals (fed vs food-deprived).
Conclusions
Our results support a mechanism of liraglutide and semaglutide in vivo to activate POMC while inhibiting NPY/AgRP neurons, which depends upon metabolic state and mirrors the pharmacokinetic profile of these compounds in vivo.




































 京公网安备 11010802027423号
京公网安备 11010802027423号