当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety and efficacy of depatuxizumab mafodotin in Japanese patients with malignant glioma: A nonrandomized, phase 1/2 trial
Cancer Science ( IF 4.5 ) Pub Date : 2021-10-05 , DOI: 10.1111/cas.15153 Yoshitaka Narita 1 , Yoshihiro Muragaki 2 , Naoki Kagawa 3 , Katsunori Asai 4 , Motoo Nagane 5 , Masahide Matsuda 6 , Keisuke Ueki 7 , Junichiro Kuroda 8 , Isao Date 9 , Hiroyuki Kobayashi 10 , Toshihiro Kumabe 11 , Takaaki Beppu 12 , Masayuki Kanamori 13 , Shota Kasai 14 , Yasuko Nishimura 14 , Hao Xiong 15 , Christopher Ocampo 15 , Masakazu Yamada 16 , Kazuhiko Mishima 17
Cancer Science ( IF 4.5 ) Pub Date : 2021-10-05 , DOI: 10.1111/cas.15153 Yoshitaka Narita 1 , Yoshihiro Muragaki 2 , Naoki Kagawa 3 , Katsunori Asai 4 , Motoo Nagane 5 , Masahide Matsuda 6 , Keisuke Ueki 7 , Junichiro Kuroda 8 , Isao Date 9 , Hiroyuki Kobayashi 10 , Toshihiro Kumabe 11 , Takaaki Beppu 12 , Masayuki Kanamori 13 , Shota Kasai 14 , Yasuko Nishimura 14 , Hao Xiong 15 , Christopher Ocampo 15 , Masakazu Yamada 16 , Kazuhiko Mishima 17
Affiliation
|
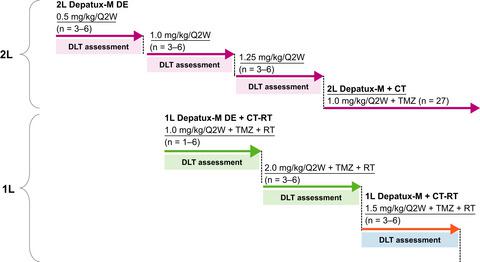
INTELLANCE-J was a phase 1/2 study of a potent antibody-drug conjugate targeting epidermal growth factor receptor (EGFR), depatuxizumab mafodotin (Depatux-M), as a second- or first-line therapy, alone or combined with chemotherapy or chemoradiotherapy in 53 Japanese patients with World Health Organization (WHO) grade III/IV glioma. In second-line arms, patients with EGFR-amplified recurrent WHO grade III/IV glioma received Depatux-M plus chemotherapy (temozolomide) or Depatux-M alone regardless of EGFR status. In first-line arms, patients with newly diagnosed WHO grade III/IV glioma received Depatux-M plus chemoradiotherapy. The study was halted following lack of survival benefit with first-line Depatux-M in the global trial INTELLANCE-1. The primary endpoint was 6-month progression-free survival (PFS) in patients with EGFR-amplified tumors receiving second-line Depatux-M plus chemotherapy. Common nonocular treatment-emergent adverse events (TEAEs) with both second-line and first-line Depatux-M included lymphopenia (42%, 33%, respectively), thrombocytopenia (39%, 47%), alanine aminotransferase increase (29%, 47%), and aspartate aminotransferase increase (24%, 60%); incidence of grade ≥3 TEAEs was 66% and 53%, respectively. Ocular side effects (OSEs) occurred in 93% of patients receiving second-line Depatux-M plus chemotherapy and all patients receiving second-line Depatux-M alone or first-line Depatux-M plus chemoradiotherapy. Most OSEs were manageable with dose modifications and concomitant medications. The 6-month PFS estimate was 25.6% (95% confidence interval [CI] 11.4‒42.6), and median PFS was 2.1 months (95% CI 1.9‒3.9) with second-line Depatux-M plus chemotherapy in the EGFR-amplified subgroup. This study showed acceptable safety profile of Depatux-M alone or plus chemotherapy/chemoradiotherapy in Japanese patients with WHO grade III/IV glioma. The study was registered at ClinicalTrials.gov (NCT02590263).
中文翻译:

depatuxizumab mafodotin 在日本恶性胶质瘤患者中的安全性和有效性:一项非随机、1/2 期试验
INTELLANCE-J 是一项针对表皮生长因子受体 (EGFR) depatuxizumab mafodotin (Depatux-M) 的强效抗体-药物偶联物作为二线或一线治疗的 1/2 期研究,单独或与化疗或53 例日本世界卫生组织 (WHO) III/IV 级胶质瘤患者的放化疗。在二线治疗组中,EGFR 扩增的复发性 WHO III/IV 级胶质瘤患者接受 Depatux-M 加化疗(替莫唑胺)或单独 Depatux-M,无论 EGFR 状态如何。在一线治疗组中,新诊断的 WHO III/IV 级胶质瘤患者接受了 Depatux-M 加放化疗。在全球试验 INTELLANCE-1 中,由于缺乏一线 Depatux-M 的生存获益,该研究被停止。主要终点是接受二线 Depatux-M 联合化疗的 EGFR 扩增肿瘤患者的 6 个月无进展生存期 (PFS)。二线和一线 Depatux-M 的常见非眼部治疗出现的不良事件 (TEAE) 包括淋巴细胞减少(分别为 42%、33%)、血小板减少(39%、47%)、丙氨酸转氨酶升高(29%, 47%),天冬氨酸转氨酶增加 (24%, 60%);≥3 级 TEAE 的发生率分别为 66% 和 53%。93% 接受二线 Depatux-M 加化疗的患者和所有接受二线 Depatux-M 单独或一线 Depatux-M 加放化疗的患者发生眼部副作用 (OSE)。大多数 OSE 可以通过剂量调整和伴随药物控制。6 个月 PFS 估计值为 25.6%(95% 置信区间 [CI] 11.4-42.6),在 EGFR 扩增亚组中,二线 Depatux-M 加化疗的中位 PFS 为 2.1 个月(95% CI 1.9-3.9)。该研究显示,在日本 WHO III/IV 级胶质瘤患者中,单独使用 Depatux-M 或联合化疗/放化疗的安全性是可接受的。该研究已在 ClinicalTrials.gov (NCT02590263) 上注册。
更新日期:2021-12-06
中文翻译:

depatuxizumab mafodotin 在日本恶性胶质瘤患者中的安全性和有效性:一项非随机、1/2 期试验
INTELLANCE-J 是一项针对表皮生长因子受体 (EGFR) depatuxizumab mafodotin (Depatux-M) 的强效抗体-药物偶联物作为二线或一线治疗的 1/2 期研究,单独或与化疗或53 例日本世界卫生组织 (WHO) III/IV 级胶质瘤患者的放化疗。在二线治疗组中,EGFR 扩增的复发性 WHO III/IV 级胶质瘤患者接受 Depatux-M 加化疗(替莫唑胺)或单独 Depatux-M,无论 EGFR 状态如何。在一线治疗组中,新诊断的 WHO III/IV 级胶质瘤患者接受了 Depatux-M 加放化疗。在全球试验 INTELLANCE-1 中,由于缺乏一线 Depatux-M 的生存获益,该研究被停止。主要终点是接受二线 Depatux-M 联合化疗的 EGFR 扩增肿瘤患者的 6 个月无进展生存期 (PFS)。二线和一线 Depatux-M 的常见非眼部治疗出现的不良事件 (TEAE) 包括淋巴细胞减少(分别为 42%、33%)、血小板减少(39%、47%)、丙氨酸转氨酶升高(29%, 47%),天冬氨酸转氨酶增加 (24%, 60%);≥3 级 TEAE 的发生率分别为 66% 和 53%。93% 接受二线 Depatux-M 加化疗的患者和所有接受二线 Depatux-M 单独或一线 Depatux-M 加放化疗的患者发生眼部副作用 (OSE)。大多数 OSE 可以通过剂量调整和伴随药物控制。6 个月 PFS 估计值为 25.6%(95% 置信区间 [CI] 11.4-42.6),在 EGFR 扩增亚组中,二线 Depatux-M 加化疗的中位 PFS 为 2.1 个月(95% CI 1.9-3.9)。该研究显示,在日本 WHO III/IV 级胶质瘤患者中,单独使用 Depatux-M 或联合化疗/放化疗的安全性是可接受的。该研究已在 ClinicalTrials.gov (NCT02590263) 上注册。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号