Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregation of Halloysite Nanotubes in the Presence of Multivalent Ions and Ionic Liquids
Langmuir ( IF 3.7 ) Pub Date : 2021-10-04 , DOI: 10.1021/acs.langmuir.1c01949
Bojana Katana 1 , Dóra Takács 1 , Adél Szerlauth 1 , Szilárd Sáringer 1 , Gábor Varga 2 , Andrej Jamnik 3 , Felix D Bobbink 4 , Paul J Dyson 4 , Istvan Szilagyi 1
Langmuir ( IF 3.7 ) Pub Date : 2021-10-04 , DOI: 10.1021/acs.langmuir.1c01949
Bojana Katana 1 , Dóra Takács 1 , Adél Szerlauth 1 , Szilárd Sáringer 1 , Gábor Varga 2 , Andrej Jamnik 3 , Felix D Bobbink 4 , Paul J Dyson 4 , Istvan Szilagyi 1
Affiliation
|
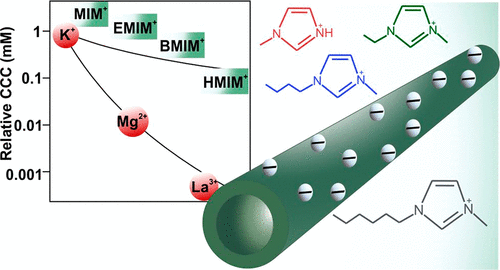
Colloidal stability was investigated in two types of particle systems, namely, with bare (h-HNT) and polyimidazolium-functionalized (h-HNT–IP-2) alkali-treated halloysite nanotubes in solutions of metal salts and ionic liquids (ILs). The valence of the metal ions and the number of carbon atoms in the hydrocarbon chain of the IL cations (1-methylimidazolium (MIM+), 1-ethyl-3-methylimidazolium (EMIM+), 1-butyl-3-methylimidazolium (BMIM+), and 1-hexyl-3-methylimidazolium (HMIM+)) were altered in the measurements. For the bare h-HNT with a negative surface charge, multivalent counterions destabilized the dispersions at low values of critical coagulation concentration (CCC) in line with the Schulze–Hardy rule. In the presence of ILs, significant adsorption of HMIM+ took place on the h-HNT surface, leading to charge neutralization and overcharging at appropriate concentrations. A weaker affinity was observed for MIM+, EMIM+, and BMIM+, while they adsorbed on the particles to different extents. The order HMIM+ < BMIM+ < EMIM+ < MIM+ was obtained for the CCCs of h-HNT, indicating that HMIM+ was the most effective in the destabilization of the colloids. For h-HNT–IP-2 with a positive surface charge, no specific interaction was observed between the salt and the IL constituent cations and the particles, i.e., the determined charge and aggregation parameters were the same within experimental error, irrespective of the type of co-ions. These results clearly indicate the relevance of ion adsorption in the colloidal stability of the nanotubes and thus provide useful information for further design of processable h-HNT dispersions.
中文翻译:

多价离子和离子液体存在下埃洛石纳米管的聚集
研究了两种类型的颗粒系统的胶体稳定性,即在金属盐和离子液体(IL)溶液中使用裸露(h-HNT)和聚咪唑功能化(h-HNT-IP-2)碱处理埃洛石纳米管。 IL 阳离子的烃链中金属离子的价态和碳原子数(1-甲基咪唑鎓 (MIM + )、1-乙基-3-甲基咪唑鎓 (EMIM + )、1-丁基-3-甲基咪唑鎓 (BMIM + ) 和 1-己基-3-甲基咪唑鎓 (HMIM + )) 在测量中发生了变化。对于表面带负电荷的裸 h-HNT,多价抗衡离子在临界凝结浓度 (CCC) 的低值下使分散体不稳定,符合 Schulze-Hardy 规则。在存在 IL 的情况下,HMIM +在 h-HNT 表面上发生显着吸附,导致适当浓度下的电荷中和和过度充电。 MIM + 、EMIM +和BMIM +的亲和力较弱,但它们不同程度地吸附在颗粒上。 h-HNT 的 CCC 的顺序为 HMIM + < BMIM + < EMIM + < MIM + ,表明 HMIM +在胶体去稳定方面最有效。对于具有正表面电荷的 h-HNT-IP-2,在盐和 IL 组成阳离子以及颗粒之间没有观察到特异性相互作用,即,无论类型如何,所确定的电荷和聚集参数在实验误差内是相同的共离子。 这些结果清楚地表明了离子吸附与纳米管胶体稳定性的相关性,从而为进一步设计可加工的 h-HNT 分散体提供了有用的信息。
更新日期:2021-10-12
中文翻译:

多价离子和离子液体存在下埃洛石纳米管的聚集
研究了两种类型的颗粒系统的胶体稳定性,即在金属盐和离子液体(IL)溶液中使用裸露(h-HNT)和聚咪唑功能化(h-HNT-IP-2)碱处理埃洛石纳米管。 IL 阳离子的烃链中金属离子的价态和碳原子数(1-甲基咪唑鎓 (MIM + )、1-乙基-3-甲基咪唑鎓 (EMIM + )、1-丁基-3-甲基咪唑鎓 (BMIM + ) 和 1-己基-3-甲基咪唑鎓 (HMIM + )) 在测量中发生了变化。对于表面带负电荷的裸 h-HNT,多价抗衡离子在临界凝结浓度 (CCC) 的低值下使分散体不稳定,符合 Schulze-Hardy 规则。在存在 IL 的情况下,HMIM +在 h-HNT 表面上发生显着吸附,导致适当浓度下的电荷中和和过度充电。 MIM + 、EMIM +和BMIM +的亲和力较弱,但它们不同程度地吸附在颗粒上。 h-HNT 的 CCC 的顺序为 HMIM + < BMIM + < EMIM + < MIM + ,表明 HMIM +在胶体去稳定方面最有效。对于具有正表面电荷的 h-HNT-IP-2,在盐和 IL 组成阳离子以及颗粒之间没有观察到特异性相互作用,即,无论类型如何,所确定的电荷和聚集参数在实验误差内是相同的共离子。 这些结果清楚地表明了离子吸附与纳米管胶体稳定性的相关性,从而为进一步设计可加工的 h-HNT 分散体提供了有用的信息。

































 京公网安备 11010802027423号
京公网安备 11010802027423号